Research Article |
|
Corresponding author: Kazuaki Tanaka ( k-tanaka@hirosaki-u.ac.jp ) Academic editor: Huzefa Raja
© 2022 Ryosuke Sugita, Kazuaki Tanaka.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Sugita R, Tanaka K (2022) Thyridium revised: Synonymisation of Phialemoniopsis under Thyridium and establishment of a new order, Thyridiales. MycoKeys 86: 147-176. https://doi.org/10.3897/mycokeys.86.78989
|
Abstract
The genus Thyridium, previously known as a saprobic or hemibiotrophic ascomycete on various plants, was revised taxonomically and phylogenetically. Sequences of the following six regions, that is, the nuclear ribosomal internal transcribed spacer (ITS) region, the large subunit (LSU) of rDNA, the second largest RNA polymerase II subunit (rpb2) gene, translation elongation factor 1-alpha (tef1) gene, the actin (act) gene, and the beta-tubulin (tub2) gene, were generated for molecular phylogenetic analyses of species of this genus. Phialemoniopsis, a genus encompassing medically important species, is synonymised with Thyridium based on molecular evidence and morphological similarities in their asexual characters. The generic concept for Thyridium is expanded to include species possessing both coelomycetous and hyphomycetous complex asexual morphs. In addition to type species of Thyridium, T. vestitum, nine species were accepted in Thyridium upon morphological comparison and molecular phylogenetic analyses in this study. All seven species of Phialemoniopsis were treated as members of the genus Thyridium and new combinations were proposed. A bambusicolous fungus, Pleospora punctulata, was transferred to Thyridium, and an epitype is designated for this species. A new species, T. flavostromatum, was described from Phyllostachys pubescens. The family Phialemoniopsidaceae, proposed as a familial placement for Phialemoniopsis, was regarded as a synonym of Thyridiaceae. A new order, Thyridiales, was established to accommodate Thyridiaceae; it forms a well-supported, monophyletic clade in Sordariomycetes.
Keywords
Ascomycota, Phialemoniopsidaceae, phylogeny, Sordariomycetes, taxonomy, Thyridiaceae
Introduction
Thyridium was originally established to accommodate species with cylindrical, uniseriate, 8-spored asci and muriform, dark-coloured, ascospores (
Molecular information on Thyridium species is available only for two non-type strains (CBS 113027, CBS 125582) of the type species T. vestitum (
The genus Phialemoniopsis was placed in Phialemoniopsidaceae (Diaporthomycetidae family incertae sedis, Sordariomycetes;
In our ongoing taxonomic study of sordariomycetous fungi in Japan, several new specimens of Thyridium-like sexual morphs were collected. Single ascospore isolates from these specimens formed typical Phialemoniopsis-like asexual morphs in culture, suggesting that both genera are closely related. This study aims to reveal the taxonomic and phylogenetic relationships between Thyridium and Phialemoniopsis, and to clarify their ordinal position in Sordariomycetes.
Material and method
Isolation and morphological observation
All materials were obtained from Japan. Morphological characteristics were observed in preparations mounted in distilled water by differential interference and phase contrast microscopy (Olympus BX53) using images captured with an Olympus digital camera (DP21). All specimens were deposited in the herbarium at Hirosaki University (
Phylogenetic analyses
DNA was extracted from four isolates using the ISOPLANT II kit (Nippon Gene, Tokyo, Japan) following the manufacturer’s instructions. The following loci were amplified and sequenced: the internal transcribed spacer (ITS) region with primers ITS1 and ITS4 (
Isolates and GenBank accessions of sequences used in the phylogenetic analyses of Sordariomycetes (Fig.
| Taxon | Isolatea | Statusb | GenBank accession numbersa | Ref.c | ||
|---|---|---|---|---|---|---|
| LSU | rpb2 | tef1 | ||||
| Acrodictys aquatica | MFLUCC 18-0356 | HT | MG835712 | – | – | 47 |
| Acrodictys bambusicola | HSAUP myr9510 | KX033564 | – | – | 44 | |
| Annulatascus velatisporus | A70 18 | AY316354 | – | – | 3 | |
| Annulusmagnus triseptatus | CBS 128831 | GQ996540 | JQ429258 | – | 25, 29 | |
| Ascitendus austriascus | CBS 131685 | GQ996539 | JQ429257 | – | 25, 29 | |
| Atractospora reticulata | CBS 127884 | HT | KT991660 | KT991649 | – | 41 |
| Atractospora thailandensis | KUMCC 16-0067 | HT | MF374362 | MF370951 | MF370962 | 45 |
| Barbatosphaeria arboricola | CBS 127689 | HT | KM492862 | KM492901 | – | 38 |
| Barbatosphaeria barbirostris | CBS 121149 | EF577059 | KM492903 | – | 18, 38 | |
| Barbatosphaeria varioseptata | CBS 137797 | HT | KM492869 | KM492907 | – | 38 |
| Barrmaelia rhamnicola | CBS 142772 | ET | MF488990 | MF488999 | MF489009 | 52 |
| Bombardia bombarda | AFTOL-ID 967 | DQ470970 | DQ470923 | DQ471095 | 14 | |
| Calosphaeria pulchella | CBS 115999 | IT | AY761075 | GU180661 | FJ238421 | 8, 27 |
| Camarops microspora | CBS 649.92 | AY083821 | DQ470937 | – | 13, 14 | |
| Camarotella costaricensis | MM-149 | KX430484 | KX451954 | KX451982 | 43 | |
| Cancellidium cinereum | MFLUCC 18-0424 | HT | MT370363 | MT370486 | MT370488 | 57 |
| Cancellidium griseonigrum | MFLUCC 17-2117 | HT | MT370364 | MT370487 | – | 57 |
| Ceratolenta caudata | CBS 125234 | HT | JX066704 | JX066699 | – | 33 |
| PRM 899855 | JX066705 | – | – | 33 | ||
| Chaetosphaeria ciliata | ICMP 18253 | GU180637 | GU180659 | – | 27 | |
| Chaetosphaeria curvispora | ICMP 18255 | GU180636 | GU180655 | – | 27 | |
| Cryptadelphia groenendalensis | SH12 | EU528007 | – | – | 20 | |
| SMH3767 | EU528001 | – | – | 20 | ||
| Diaporthe phaseolorum | NRRL 13736 | U47830 | – | – | 1 | |
| Distoseptispora obpyriformis | MFLUCC 17-1694 | HT | MG979764 | MG988415 | MG988422 | 48 |
| Distoseptispora rostrata | MFLUCC 16-096 | HT | MG979766 | MG988417 | MG988424 | 48 |
| Endoxyla operculata | UAMH 11085 | JX460992 | KY931927 | – | 34, 49 | |
| Entosordaria perfidiosa | CBS 142773 | ET | MF488993 | MF489003 | MF489012 | 52 |
| Fluminicola aquatica | MFLUCC 15-0962 | HT | MF374366 | – | MF370960 | 45 |
| Fluminicola saprotrophitica | MFLUCC 15-0976 | HT | MF374367 | MF370954 | MF370956 | 45 |
| Gnomonia gnomon | CBS 199.53 | AF408361 | DQ470922 | DQ471094 | 2, 14 | |
| Jobellisia fraterna | SMH2863 | AY346285 | – | – | 4 | |
| Jobellisia luteola | SMH2753 | AY346286 | – | – | 4 | |
| Lanspora coronata | AFTOL-ID 736 | U46889 | DQ470899 | – | 14 | |
| Lasiosphaeria ovina | SMH4605 | AY436413 | AY600284 | DQ836908 | 6, 7, 16 | |
| Lentomitella cirrhosa | ICMP 15131 | ET | AY761085 | KM492911 | – | 11, 38 |
| Lentomitella crinigera | CBS 138678 | KY931811 | – | – | 49 | |
| Linocarpon livistonae | HKUM 6520 | DQ810205 | DQ810248 | – | 10 | |
| Magnaporthe salvinii | M 21 | JF414887 | – | JF710406 | 28 | |
| Magnaporthiopsis agrostidis | CBS 142740 | HT | KT364754 | – | KT364756 | 37 |
| Melanconis stilbostoma | CBS 109778 | AF408374 | EU219299 | EU221886 | 2 | |
| Myrmecridium montsegurinum | JF 13180 | HT | KT991664 | KT991654 | – | 41 |
| Myrmecridium schulzeri | CBS 100.54 | EU041826 | – | – | 17 | |
| Myrmecridium thailandicum | CBS 136551 | HT | KF777222 | – | – | 30 |
| Neolinocarpon enshiense | HKUCC 2983 | DQ810221 | DQ810244 | – | 10 | |
| Neolinocarpon globosicarpum | HKUCC 1959 | DQ810224 | DQ810245 | – | 10 | |
| Ophiostoma piliferum | CBS 158.74 | DQ470955 | DQ470905 | DQ471074 | 14 | |
| Ophiostoma stenoceras | CBS 139.51 | DQ836904 | DQ836891 | DQ836912 | 16 | |
| Papulosa amerospora | AFTOL-ID 748 | DQ470950 | DQ470901 | DQ471069 | 14 | |
| Pararamichloridium caricicola | CBS 145069 | HT | MK047488 | – | – | 46 |
| Pararamichloridium livistonae | CBS 143166 | HT | MG386084 | – | – | 54 |
| Pararamichloridium verrucosum | CBS 128.86 | HT | MH873621 | – | – | 56 |
| Phaeoacremonium fraxinopennsylvanica | M.R. 3064 | HQ878595 | HQ878609 | – | 26 | |
| Phaeoacremonium novae-zealandiae | CBS 110156 | HT | AY761081 | – | – | 8 |
| Phomatospora bellaminuta | AFTOL-ID 766 | FJ176857 | FJ238345 | – | 23 | |
| Phomatospora biseriata | MFLUCC 14-0832A | KX549448 | – | – | 51 | |
| Phyllachora graminis | TH-544 | KX430508 | – | – | 43 | |
| Pleurostoma ootheca | CBS 115329 | IT | AY761079 | HQ878606 | FJ238420 | 8, 23, 26 |
| Pseudostanjehughesia aquitropica | MFLUCC 16-0569 | HT | MF077559 | – | MF135655 | 53 |
| Pseudostanjehughesia lignicola | MFLUCC 15-0352 | HT | MK849787 | MN124534 | MN194047 | 55 |
| Pyricularia borealis | CBS 461.65 | DQ341511 | – | – | 24 | |
| Pyricularia bothriochloae | CBS 136427 | HT | KF777238 | – | – | 30 |
| Rhamphoria delicatula | CBS 132724 | FJ617561 | JX066702 | – | 22, 33 | |
| Rhamphoria pyriformis | CBS 139024 | MG600397 | MG600401 | – | 50 | |
| Rubellisphaeria abscondita | CBS 132078 | HT | KT991666 | KT991657 | – | 41 |
| Sordaria fimicola | CBS 723.96 | AY780079 | DQ368647 | – | 9, 19 | |
| Spadicoides bina | CBS 137794 | KY931824 | KY931851 | – | 49 | |
| Sporidesmium minigelatinosa | NN 47497 | DQ408567 | DQ435090 | – | 12 | |
| Sporidesmium parvum | HKUCC 10836 | DQ408558 | – | – | 12 | |
| Thyridium cornearis | CBS 131711 | HT | KJ573450 | – | LC382144 | 36 |
| Thyridium curvatum | CBS 490.82 | HT | AB189156 | – | LC382142 | 15 |
| Thyridium endophyticum | ACCC 38980 | HT | KT799560 | – | – | 42 |
| Thyridium flavostromatum | KT 3891 = MAFF 247509 | HT | LC655963 | LC655967 | LC655971 | This study |
| Thyridium hongkongense | HKU39 | HT | KJ573447 | – | – | 36 |
| Thyridium limonesiae | CBS 146752 | HT | MW050976 | – | – | 58 |
| Thyridium oculorum | CBS 110031 | HT | KJ573449 | – | LC382145 | 36 |
| Thyridium pluriloculosum | CBS 131712 | HT | HE599271 | – | LC382141 | 32 |
| KT 3803 = MAFF 247508 | LC655964 | LC655968 | LC655972 | This study | ||
| Thyridium punctulatum | KT 1015 = MAFF 239669 | LC655965 | LC655969 | LC655973 | This study | |
| KT 3905 = MAFF 247510 | ET | LC655966 | LC655970 | LC655974 | This study | |
| Thyridium vestitum | CBS 113027 | AY544671 | DQ470890 | DQ471058 d | 5, 14 | |
| CBS 125582 | MH875182 | – | – | 56 | ||
| Tirisporella beccariana | BCC 36737 | JQ655450 | – | – | 39 | |
| Tirisporella bisetulosus | BCC 00018 | EF622230 | – | – | 21 | |
| Wongia griffinii | BRIP 60377 | KU850470 | – | KU850466 | 40 | |
| Woswasia atropurpurea | CBS 133167 | HT | JX233658 | JX233659 | – | 31 |
| Xylochrysis lucida | CBS 135996 | HT | KF539911 | KF539913 | – | 35 |
| Xylolentia brunneola | PRA-13611 | HT | MG600398 | MG600402 | – | 50 |
Primary analysis of LSU-rpb2-tef1 sequences from 88 strains of Sordariomycetes (Table
Isolates and GenBank accessions of sequences used in the phylogenetic analyses of Thyridium species (Fig.
| Taxon | Isolatea | Substrate/Host | Statusb | GenBank accession numbersa | Ref.c | ||
|---|---|---|---|---|---|---|---|
| ITS | act | tub2 | |||||
| Thyridium cornearis | CBS 131711 | human corneal fluid | HT | KJ573445 | HE599252 | HE599301 | 1, 2 |
| UTHSC 06-1465 | shin aspirate | HE599285 | HE599253 | HE599302 | 2 | ||
| Thyridium curvatum | CBS 490.82 | skin lesion | HT | AB278180 | HE599258 | HE599307 | 2 |
| UTHSC R-3447 | human eye | HE599291 | HE599259 | HE599308 | 2 | ||
| Thyridium endophyticum | ACCC 38979 | lower stem of Luffa cylindrica (endophyte) | KT799556 | KT799553 | KT799562 | 4 | |
| ACCC 38980 | lower stem of Luffa cylindrica (endophyte) | HT | KT799557 | KT799554 | KT799563 | 4 | |
| Thyridium flavostromatum | KT 3891 = MAFF 247509 | dead twigs of Phyllostachys pubescens | HT | LC655959 | LC655979 | LC655975 | This study |
| Thyridium hongkongense | HKU39 | the right forearm nodule biopsy of a human | HT | KJ573442 | KJ573452 | KJ573457 | 3 |
| Thyridium limonesiae | CBS 146752 | Skin nodule | HT | MW050977 | MW349126 | MW048608 | 6 |
| Thyridium oculorum | CBS 110031 | human keratitis | HT | KJ573444 | HE599247 | HE599296 | 2, 3 |
| UTHSC 05-2527 | peritoneal dialysis catheter | HE599281 | HE599249 | HE599298 | 2 | ||
| Thyridium pluriloculosum | CBS 131712 | human toe nail | HT | HE599286 | HE599254 | HE599303 | 2 |
| KT 3803 = MAFF 247508 | dead wood of Betula maximowicziana | HT | LC655960 | LC655980 | LC655976 | This study | |
| UTHSC 09-3589 | synovial fluid | HE599287 | HE599255 | HE599304 | 2 | ||
| Thyridium punctulatum | KT 1015 = MAFF 239669 | dead culms of Phyllostachys pubescens | LC655961 | LC655981 | LC655977 | This study | |
| KT 3905 = MAFF 247510 | dead twigs of Phyllostachys nigra var. nigra | ET | LC655962 | LC655982 | LC655978 | This study | |
| Thyridium vestitum | CBS 125582 | MH863721 | – | – | 5 | ||
Phylogenetic analyses were conducted using maximum-likelihood (ML) and Bayesian methods. The optimum substitution models for each dataset were estimated using Kakusan4 software (
Result
Phylogeny
For primary analysis, ML and Bayesian phylogenetic trees were generated using an aligned sequence dataset comprising of LSU (1,205 base pairs), rpb2 (1,059 bp) and tef1 (954 bp). Of the 3,218 characters included in the alignment, 1,478 were variable and 1,686 were conserved. This combined dataset provided higher confidence values for ordinal and familial classification than those of individual gene trees, with 25 orders and three families (order unknown) being reconstructed in Sordariomycetes (Fig.
Maximum-likelihood tree of Sordariomycetes based on combined LSU, rpb2 and tef1 sequence. ML bootstrap proportion (BP) greater than 70% and Bayesian posterior probabilities (PP) above 0.95 are presented at the nodes as ML BP/Bayesian PP and a node not present in the Bayesian analysis is shown with ‘x’. A hyphen (‘-’) indicates values lower than 70% BP or 0.95 PP. Ex-holotype, isotype, paratype and epitype strains are shown in bold and the newly obtained sequences are shown in red. Strains previously described as Phialemoniopsis species are marked with a blue circle. The scale bar represents nucleotide substitutions per site.
For secondary analysis, ML and Bayesian phylogenetic trees were generated using sequences of ITS (483 bp), act (646 bp), tub2 (375 bp), and a combined dataset of these three regions (1,504 bp). The selected substitution models for each region were as follows: J2ef+G for ITS, F81+H for the first and second codon positions of act, J2+G for the third codon position of act, K80+H for the first codon positions of tub2, JC69+H for the second codon position of tub2 and TN93+H for the third codon position of tub2. The ML trees with the highest log likelihood (–1172.0198 in ITS, –1196.6012 in act, –859.37115 in tub2 and –3315.7254 in ITS-act-tub2) are shown in Fig.
Maximum-likelihood tree of Thyridium species based on each ITS (A), act (B), tub2 (C) and combined sequences (ITS-act-tub2; D). ML bootstrap proportion (BP) greater than 70% and Bayesian posterior probabilities (PP) above 0.95 are presented at the nodes as ML BP/Bayesian PP. A hyphen (‘-’) indicates values lower than 70% BP or 0.95 PP and a node not present in the Bayesian analysis is shown with ‘x’. Ex-holotype and epitype strains are shown in bold and the newly obtained sequences are shown in red. Strains previously as Phialemoniopsis species are marked with a blue circle. The scale bars represent nucleotide substitutions per site.
Taxonomy
A new order, Thyridiales, is introduced to accommodate Thyridiaceae because its lineage is phylogenetically and morphologically distinct from any known orders in Sordariomycetes. We concluded Thyridium and Phialemoniopsis to be congeneric based on their morphological similarities and phylogenetic relatedness. An expanded generic circumscription of Thyridium that integrates the generic concept of Phialemoniopsis is provided below. One new species and eight new combinations of Thyridium are proposed.
Thyridiales , ord. nov.
Type family
Thyridiaceae J.Z. Yue & O.E. Erikss., Syst. Ascom. 6(2): 233 (1987).
Sexual morph
Stromata scattered to grouped. Ascomata perithecial, subglobose to ampulliform. Ostiolar neck cylindrical, periphysate. Paraphyses numerous, unbranched, cylindrical, hyaline. Asci unitunicate, cylindrical, with an apical annulus, pedicellate. Ascospores obovoid to ellipsoid, muriform, hyaline to brown.
Asexual morph
Coelomycetous asexual morph: Conidiomata pycnidial, globose to subglobose. Conidiogenous cells phialidic. Conidia ellipsoidal to obovoid, aseptate, hyaline. Hyphomycetous synasexual morph: Colonies effuse or sporodochial. Conidiophores micronematous, mononematous, simple or branched, hyaline, thin-walled. Conidiogenous cells phialidic. Conidia ellipsoidal to allantoid, aseptate, hyaline.
Notes
Thyridiaceae has been treated as incertae sedis in Sordariomycetes (
Thyridiaceae
Phialemoniopsidaceae K.D. Hyde & Hongsanan, [as Phialemoniopsaceae] Fungal Divers. 107: 95 (2021).
Type genus
Thyridium Nitschke, Pyrenomyc. Germ. 1: 110 (1867).
Notes
Phialemoniopsidaceae is considered a synonym of Thyridiaceae because Phialemoniopsis, the type genus of Phialemoniopsidaceae, was revealed congeneric with Thyridium and is placed in the synonymy of the latter genus in this study. The type genera of both families, that is, Thyridium and Phialemoniopsis, share many morphological features in their asexual states, as noted below.
Thyridium
Melanospora subgen. Bivonella Sacc., Syll. fung. (Abellini) 2: 464 (1883).
Bivonella (Sacc.) Sacc., Syll. fung. (Abellini) 9: 989 (1891).
Pleurocytospora Petr., Annls mycol. 21: 256 (1923).
Sinosphaeria J.Z. Yue & O.E. Erikss., Syst. Ascom. 6: 231 (1987).
Phialemoniopsis Perdomo, Dania García, Gené, Cano & Guarro, Mycologia 105: 408 (2013).
Type species
Thyridium vestitum (Fr.) Fuckel, Jb. nassau.Ver. Naturk. 23–24: 195 (1870) [1869–70].
Sexual morph
Stromata scattered to grouped, subepidermal to erumpent, yellowish to dark brown, red in KOH or not changing. Ascomata perithecial, subglobose to ampulliform, single to grouped, immersed in stromata to erumpent through host surface. Ascomatal wall composed of several layers of polygonal, dark brown cells. Ostiolar neck cylindrical, short or long, separated or convergent in upper stromata, periphysate. Paraphyses numerous, septate, unbranched, cylindrical, hyaline. Asci unitunicate, cylindrical, broadly rounded at the apex, with a pronounced non-amyloid apical annulus, pedicellate. Ascospores obovoid or ellipsoid, smooth, pale brown to brown, with several transverse and 0–3 longitudinal or oblique septa.
Asexual morph
Coelomycetous and/or hyphomycetous morphs formed. Coelomycetous asexual morph: Conidiomata pycnidial, single to grouped, superficial or immersed in stromata, globose to subglobose, composed of polygonal to prismatic cells, often becoming cup-shaped when mature, surrounded by setose hyphae. Conidiomatal wall composed of several layers of polygonal, dark brown cells. Ostiolar neck cylindrical, central, periphysate. Setose hyphae erect, usually unbranched, septate, cylindrical, with slightly pointed or blunt tips, hyaline to pale brown, smooth-walled. Conidiophores hyaline, thin-walled, simple or irregularly branched, with branches bearing a small group of phialides terminally. Phialides swollen at the base, tapering at the tip, hyaline. Conidia obovoid to oblong, with a slightly apiculate base, hyaline, smooth-walled, in slimy masses. Hyphomycetous synasexual morph: Colonies effuse or sporodochial. Conidiophores micronematous, mononematous, hyaline, thin-walled, simple or irregularly branched, with branches bearing a small group of phialides terminally. Phialides swollen at the base, tapering at the tip, hyaline. Adelophialides absent or rarely present. Conidia ellipsoidal to allantoid, with a slightly apiculate base, hyaline, smooth-walled, in slimy head. Chlamydospores absent or rarely present, hyaline to pale brown, thick- and rough-walled.
Notes
The newly obtained Thyridium collections formed synasexual morphs, coelomycetous and hyphomycetous, in culture that were similar to those of Phialemoniopsis, having coelomycetous and/or hyphomycetous conidial states in culture (
We accept both Bivonella and Sinosphaeria as synonyms of Thyridium, as proposed in previous studies (
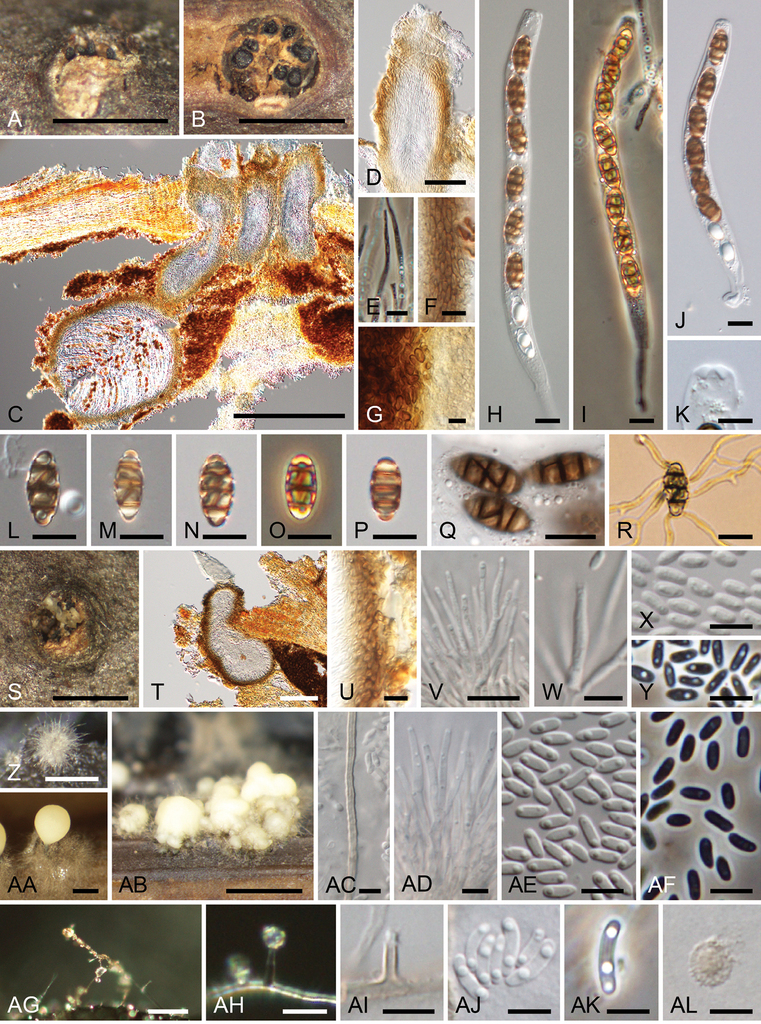
Thyridium flavostromatum (A–S KT 3891 =
Thyridium flavostromatum , sp. nov.
Holotype
Japan, Yamaguchi, Nagato, Misumikami, near Kusaritoge, on dead twigs of Phyllostachys pubescens, 26 March 2018, K. Tanaka, K. Arayama and R. Siguta, KT 3891 (
Etymology
The name refers to yellowish stromata.
Sexual morph
Stromata scattered to grouped, subepidermal, becoming erumpent to superficial, 0.7–1.4 mm long, 0.4–0.7 mm wide, yellowish to dark brown, red in 2% KOH. Ascomata perithecial, subglobose to ampulliform, mostly 2–6 grouped, 190–240 µm high, 200–220 µm diam., immersed in stromata to erumpent through host surface. Ascomatal wall 15–23 µm thick, composed of 5–8 layers of polygonal, 2.5–7 × 1.5–3.5 µm, dark brown cells. Ostiolar neck central, cylindrical, 80–140 µm long, 55–90 µm wide, periphysate. Paraphyses numerous, septate, unbranched, cylindrical, 50–105 µm long. Asci unitunicate, cylindrical, 62.5–90 × 6.5–10 µm (av. 78.7 × 7.8 µm, n = 30), broadly rounded at the apex, with a pronounced non-amyloid apical annulus, short-stalked (5–17.5 µm long), with 8 ascospores. Ascospores obovoid to ellipsoid, smooth, hyaline to pale brown, with 3 transverse and 0–2 vertical septa, 9.5–14 × 5–7.5 µm (av. 11.3 × 5.8 µm, n = 50), l/w 1.4–2.5 (av. 2.0, n = 50).
Asexual morph (nature)
Not observed.
Asexual morph (culture)
Hyphomycetous asexual morph formed. Conidiophores micronematous, mononematous, hyaline, thin-walled, simple or irregularly branched, with branches bearing a group of 2–3 phialides terminally. Phialides swollen at the base, tapering at the tip, hyaline, 3–6 × 1–1.5 µm. Adelophialides rarely present. Conidia ellipsoidal to allantoid, with a slightly apiculate base, hyaline, smooth-walled, 2–7 × 1–2.5 µm (av. 4.1 × 1.6 µm, n = 50). Chlamydospores rarely present, solitary, 3.5–6.5 µm diam., hyaline to pale brown, thick- and rough-walled.
Culture characteristics
Colonies on MEA at 25 °C attained 28–29 mm diam. after a week in the dark, whitish. On OA attained 35–37 mm diam., whitish. On PDA attained 28–31 mm diam., whitish to buff (45;
Notes
Phylogenetic analyses based on ITS, act, and tub2 sequences suggested that T. flavostromatum was closely related to T. curvatum, T. hongokgense and T. limonesiae (Fig.
Thyridium pluriloculosum , comb. nov.
Basionym
Phialemoniopsis pluriloculosa Perdomo, Dania García, Gené, Cano & Guarro, Mycologia 105: 412 (2013).
Holotype
USA, Nevada, human toe nail, D.A. Sutton, CBS H-20782, living culture CBS 131712 = UTHSC 04–7 = FMR 11070 (not seen).
Sexual morph
Stromata scattered to grouped, pulvinate, circular to elliptical in outline, elevated beyond bark surface forming pustules, 0.6–0.7 mm high, 0.9–1.0 mm diam., dark brown to black. Ascomata perithecial, subglobose to ampulliform, 4–8 grouped, 700–780 µm high, 220–280 µm diam., immersed in stromata. Ascomatal wall 17–25 µm thick, composed of 7–10 layers of polygonal, 4–6.5 × 2–4 µm, dark brown cells. Ostiolar neck central, cylindrical, 400–430 µm long, 100–110 µm wide, periphysate. Paraphyses septate, unbranched, cylindrical, 92.5–110 µm long, 3.5–5.5 µm wide. Asci unitunicate, cylindrical, 110–175 × 9–12.5 µm (av. 145.6 × 10.3 µm, n = 15), broadly rounded at the apex, with a pronounced non-amyloid apical annulus, pedicellate (12.5–27.5 µm long), with 8 ascospores. Ascospores fusiform to ellipsoid, smooth, brown, with 3 transverse and 0–2 oblique or vertical septa, 13.5–18 × 6–8 µm (av. 15.5 × 7.3 µm, n = 50), l/w 1.7–2.6 (av. 2.1, n = 50).
Asexual morph (nature)
Conidiomata pycnidial, globose to subglobose, grouped, 220–300 µm high, 90–150 µm diam., immersed in stromata. Conidiomatal wall 8–18 µm thick, composed of 3–5 layers of polygonal, 3–4.5 × 2.5–4 µm, dark brown cells. Ostiolar neck central, cylindrical, 80–110 µm long, 90–110 µm wide, composed of polygonal cells, periphysate. Conidiophores hyaline, thin-walled, with branches bearing a group of 2–5 phialides terminally. Phialides tapering toward the tip, hyaline, 11–16 × 1–2 µm. Conidia ellipsoidal, with a slightly apiculate base, hyaline, smooth-walled, 3–4.5 × 1–2 µm (av. 3.7 × 1.5 µm, n = 50). Chlamydospores not observed.
Thyridium pluriloculosum (A–Y KT 3803 =
Asexual morph (culture)
Coelomycetous asexual morph: Conidiomata pycnidial, scattered, single to grouped, superficial, globose to subglobose, 180–380 µm high, mostly 80–580 µm diam., up to 1170 µm diam. when grouped, often becoming cup-shaped when mature, surrounded by setose hyphae. Conidiomatal wall composed of polygonal to prismatic, 3–4.5 × 2.5–4 µm, dark brown cells. Setose hyphae erect, usually unbranched, septate, up to 360 µm long, 2–3 µm wide, pale brown. Conidiophores hyaline, thin-walled, simple or irregularly branched, with branches bearing a group of 2–5 phialides terminally. Phialides tapering toward the tip, hyaline, 10–25 × 1–2.5 µm. Conidia ellipsoidal, with a slightly apiculate base, hyaline, smooth-walled, in slimy masses, 3–4.5 × 1–2 µm (av. 3.8 × 1.4 µm, n = 50). Hyphomycetous synasexual morph: Conidiophores micronematous, mononematous, hyaline, simple or rarely branched. Phialides slightly tapering toward the tip, 4–11 × 1–2.5 µm, hyaline. Adelophialide absent. Conidia allantoid, hyaline, smooth-walled, in slimy heads, 3–9 × 1–2.5 µm (av. 6.2 × 1.7 µm, n = 50). Chlamydospores rarely present, solitary, 3.5–6.5 µm diam., hyaline to pale brown, thick- and rough-walled.
Culture characteristics
Colonies on MEA at 25 °C attained 31–33 mm diam. after a week in the dark, whitish. On OA attained 32–36 mm diam., whitish to grey olivaceous (107). On PDA attained 32–33 mm diam., whitish to buff (45) (Fig.
Specimen examined
Japan, Aomori, Hirakawa, Hirofune, Shigabo Forest Park, on dead twigs of Betula maximowicziana, 10 October 2017, K. Tanaka, KT 3803 (
Notes
The conidia from aerial hyphae of strain KT 3803 were larger (3–9 × 1–2.5 µm) in culture than those of the original description of Thyridium pluriloculosum (3–5 × 1–2.5 µm;
In Thyridium, T. betulae has also been recorded on Betula sp. in France (
Thyridium punctulatum , comb. nov.
Basionym
Pleospora punctulata I. Hino & Katum., Icones Fungorum Bamb. Jpn.: 181 (1961).
Holotype
Japan, Shizuoka, Fuji Bamboo Garden, on dead twigs of Phyllostachys nigra var. henonis, 1 April 1958, K. Katumoto, YAM 21851.
Epitype
Japan, Yamaguchi, Hagi, Akiragi, near Chikurindoro-park, on dead twigs of Phyllostachys nigra var. nigra, 26 March 2018, K. Tanaka, K. Arayama and R. Sugita, KT 3905 (
Sexual morph
Stromata scattered to grouped, subepidermal, becoming erumpent to superficial, 0.5–1.2 mm long, 0.2–0.4 mm wide, dark brown. Ascomata perithecial, subglobose to conical, single to 2–3 grouped, 130–190 µm high, 140–230 µm diam., immersed in stromata to erumpent through host surface. Ascomatal wall 7–15 µm thick, composed of 3–5 layers of polygonal, 3–6.5 × 1–4.5 µm, dark brown cells. Ostiolar neck central, cylindrical, 37–85 µm long, 37–63 µm wide, periphysate. Paraphyses numerous, septate, unbranched, cylindrical, hyaline, 77–103 µm long. Asci unitunicate, cylindrical, 67.5–105 × 7.5–11.5 µm (av. 82.9 × 9.4 µm, n = 60), broadly rounded at the apex, with a pronounced non-amyloid apical annulus, short-stalked (3.5–11.5 µm long), with 8 ascospores. Ascospores ellipsoid to oblong, smooth, pale brown, with 3 transverse and 1–2 vertical septa, 10–15 × 5–9 µm (av. 12.8 × 7.0 µm, n = 60), l/w 1.4–2.4 (av. 1.8, n = 60).
Thyridium punctulatum (A–N, Q, R KT 3905 =
Asexual morph (nature)
Not observed.
Asexual morph (culture)
Coelomycetous asexual morph: Conidiomata pycnidial, single to grouped, superficial, globose to subglobose, 100–250 µm high, 170–620 µm diam., composed of polygonal to prismatic, 3.5–7.5 × 2.5–4 µm cells, often becoming cup-shaped when mature, surrounded by setose hyphae. Setose hyphae erect, usually unbranched, septate, up to 225 µm long, 1.5–2.5 µm wide, pale brown. Conidiophores hyaline, thin-walled, simple or irregularly branched, with branches bearing a group of 2–5 phialides terminally. Phialides swollen at the base, tapering at the tip, 7–20 × 1–3 µm, hyaline. Conidia ellipsoidal to obovoid, with a slightly apiculate base, hyaline, smooth-walled, in slimy masses, 2–3.5 × 1–2 µm (av. 2.9 × 1.4 µm, n = 50). Hyphomycetous synasexual morph: Conidiophores micronematous, mononematous, hyaline, thin-walled, simple or irregularly branched, with branches bearing a group of 2–3 phialides terminally. Phialides swollen at the base, tapering at the tip, hyaline, 3–9 × 1–2 µm. Adelophialide absent. Conidia ellipsoidal to allantoid, hyaline, smooth-walled, in slimy heads, 2.5–8 × 1–3 µm (av. 4.3 × 1.6 µm, n = 87). Chlamydospores rarely present, solitary or chained, 4–5.5 µm diam., hyaline to pale brown.
Culture characteristics
Colonies on MEA at 25 °C attained 31–32 mm diam. after a week in the dark, granulose, whitish. On OA attained 38–39 mm diam., granulose, whitish. On PDA attained 35–36 mm diam., whitish to buff (45) (Fig.
Colony characters of Thyridium species used in this study on MEA (bottom right), OA (bottom left) and PDA (upper) within 1 week at 25 °C in the dark A T. flavostromatum (culture KT 3891 = MAFF 247509) B T. pluriloculosum (culture KT 3803 = MAFF 247508) C T. punctulatum (culture KT 3905 = MAFF 247510). Scale bars: 3 cm (A–C).
Other specimen examined
Japan, Iwate, Morioka, Ueda, Campus of Iwate University, on dead culms of Phyllostachys pubescens, 17 February 2003, K. Tanaka and Y. Harada, KT 1015 (
Notes
This species has been described from Phyllostachys nigra var. henonis, as a species of Pleospora (Dothideomycetes;
Thyridium cornearis , comb. nov.
Basionym
Phialemoniopsis cornearis Perdomo, Dania García, Gené, Cano & Guarro, Mycologia 105: 408 (2013).
Thyridium curvatum , comb. nov.
Phialemoniopsis curvata (W. Gams & W.B. Cooke) Perdomo, Dania García, Gené, Cano & Guarro, Mycologia 105: 410 (2013).
Basionym
Phialemonium curvatum W. Gams & W.B. Cooke, Mycologia 75: 980 (1983).
Thyridium endophyticum , comb. nov.
Basionym
Phialemoniopsis endophytica Lei Su & Y.C. Niu, Mycol. Progr. 15: 3 (2016).
Thyridium hongkongense , comb. nov.
Basionym
Phialemoniopsis hongkongensis Tsang, Chan, Ip, Ngan, Chen, Lau, Woo, J. Clin. Microbiol. 52: 3284 (2014).
Thyridium limonesiae , comb. nov.
Basionym
Phialemoniopsis limonesiae A. Riat, L.W. Hou & Crous, Emerging Microbes & Infections 10: 403 (2021).
Thyridium oculorum , comb. nov.
Phialemoniopsis ocularis (Gené & Guarro) Perdomo, Dania García, Gené, Cano & Guarro, Mycologia 105: 411 (2013).
Basionym
Sarcopodium oculorum Gené & Guarro, J. Clin. Microbiol. 40: 3074 (2002).
Discussion
We show that the asexual genus Phialemoniopsis (established by
The genus Thyridium has been defined mainly on the basis of sexual characters (
Synonymising Phialemoniopsis under Thyridium expanded information about the asexual morphs of Thyridium. In this genus, only T. vestitum has been demonstrated to have asexual morphs by culture studies (
In Thyridium, T. endophyticum and T. curvatum have been isolated from both plants and animals (Gam and McGinnis 1983;
Epitypification of the type species of Thyridium (T. vestitum) will be a necessary issue in the future. We used sequences from two non-type strains (CBS 113027, CBS 125582) of this species for phylogenetic analyses but they did not form a monophyletic clade (Fig.
Thyridiales established here may encompass other genera and families with morphologies distinct from the genus Thyridium (Thyridiaceae). Some species of “Linocarpon” and “Neolinocarpon” are nested within the Thyridiales (Fig.
Acknowledgments
We gratefully acknowledge Y. Harada and K. Arayama for their help with the collection of fungal specimens. We thank the curator of YAM, S. Ito, who permitted us to examine type collection. This work was partially supported by grants from the Japan Society for the Promotion of Science (JSPS 19K06802).
References
- Akaike H (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control 19: 716–723. https://doi.org/10.1109/TAC.1974.1100705
- Arzanlou M, Groenewald JZ, Gams W, Braun U, Shin H-D, Crous PW (2007) Phylogenetic and morphotaxonomic revision of Ramichloridium and allied genera. Studies in Mycology 58: 57–93. https://doi.org/10.3114/sim.2007.58.03
- Bahl J (2006) Molecular evolution of three morphologically similar families in the Xylariomycetidae (Apiosporaceae, Clypeosphaeriaceae, Hyponectriaceae). PhD thesis, The University of Hong Kong. Pokfulam, Hong Kong. http://hdl.handle.net/10722/51007
- Campbell J, Shearer CA (2004) Annulusmagnus and Ascitendus, two new genera in the Annulatascaceae. Mycologia 96: 822–833. https://doi.org/10.1080/15572536.2005.11832929
- Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94: 1017–1031. https://doi.org/10.1080/15572536.2003.11833157
- Checa J, Blanco MN, Moreno G (2013) Contributions to the family Thyridiaceae. New data on Sphaeria mutabilis. Mycotaxon 125: 149–164. https://doi.org/10.5248/125.149
- Choi J, Lee Y, Chung HS, Koo JS, Yong D, Kim YS, Lee K, Chong Y (2011) Subcutaneous Phaeohyphomycosis caused by Phaeoacremonium species in a kidney transplant patient: the first case in Korea. The Korean Journal of Laboratory Medicine 31: 201–204. https://doi.org/10.3343/kjlm.2011.31.3.201
- Crous PW, Gams W, Wingfield MJ, Wyk PSV (1996) Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88: 786–796. https://doi.org/10.1080/00275514.1996.12026716
- Crous PW, Luangsa-Ard JJ, Wingfield MJ, Carnegie AJ, Hernández-Restrepo M, Lombard L, Roux J, Barreto RW, Baseia IG, Cano-Lira JF, Martín MP, Morozova OV, Stchigel AM, Summerell BA, Brandrud TE, Dima B, García D, Giraldo A, Guarro J, Gusmão LFP, Khamsuntorn P, Noordeloos ME, Nuankaew S, Pinruan U, Rodríguez-Andrade E, Souza-Motta CM, Thangavel R, van Iperen AL, Abreu VP, Accioly T, Alves JL, Andrade JP, Bahram M, Baral H-O, Barbier E, Barnes CW, Bendiksen E, Bernard E, Bezerra JDP, Bezerra JL, Bizio E, Blair JE, Bulyonkova TM, Cabral TS, Caiafa MV, Cantillo T, Colmán AA, Conceição LB, Cruz S, Cunha AOB, Darveaux BA, da Silva AL, da Silva GA, da Silva GM, da Silva RMF, de Oliveira RJV, Oliveira RL, De Souza JT, Dueñas M, Evans HC, Epifani F, Felipe MTC, Fernández-López J, Ferreira BW, Figueiredo CN, Filippova NV, Flores JA, Gené J, Ghorbani G, Gibertoni TB, Glushakova AM, Healy R, Huhndorf SM, Iturrieta-González I, Javan-Nikkhah M, Juciano RF, Jurjević Ž, Kachalkin AV, Keochanpheng K, Krisai-Greilhuber I, Li Y-C, Lima AA, Machado AR, Madrid H, Magalhães OMC, Marbach PAS, Melanda GCS, Miller AN, Mongkolsamrit S, Nascimento RP, Oliveira TGL, Ordoñez ME, Orzes R, Palma MA, Pearce CJ, Pereira OL, Perrone G, Peterson SW, Pham THG, Piontelli E, Pordel A, Quijada L, Raja HA, Rosas de Paz E, Ryvarden L, Saitta A, Salcedo SS, Sandoval-Denis M, Santos TAB, Seifert KA, Silva BDB, Smith ME, Soares AM, Sommai S, Sousa JO, Suetrong S, Susca A, Tedersoo L, Telleria MT, Thanakitpipattana D, Valenzuela-Lopez N, Visagie CM, Zapata M, Groenewald JZ (2018) Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. https://doi.org/10.3767/persoonia.2018.41.12
- Crous PW, Schumacher RK, Akulov A, Thangavel R, Hernández-Restrepo M, Carnegie AJ, Cheewangkoon R, Wingfield MJ, Summerell BA, Quaedvlieg W, Coutinho TA, Roux J, Wood AR, Giraldo A, Groenewald JZ (2019) New and Interesting Fungi. 2. Fungal Systematics and Evolution 3: 57–134. https://doi.org/10.3114/fuse.2019.03.06
- Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, van der Bank M, Swart WJ, Stchigel AM, Cano-Lira JF, Roux J, Madrid H, Damm U, Wood AR, Shuttleworth LA, Hodges CS, Munster M, de Jesús Yáñez-Morales M, Zúñiga-Estrada L, Cruywagen EM, de Hoog GS, Silvera C, Najafzadeh J, Davison EM, Davison PJN, Barrett MD, Barrett RL, Manamgoda DS, Minnis AM, Kleczewski NM, Flory SL, Castlebury LA, Clay K, Hyde KD, Maússe-Sitoe SND, Chen S, Lechat C, Hairaud M, Lesage-Meessen L, Pawłowska J, Wilk M, Śliwińska-Wyrzychowska A, Mętrak M, Wrzosek M, Pavlic-Zupanc D, Maleme HM, Slippers B, Mac Cormack MP, Archuby DI, Grünwald NJ, Tellería MT, Dueñas M, Martín MP, Marincowitz S, de Beer ZW, Perez CA, Gené J, Marin-Felix Y, Groenewald JZ (2013) Fungal Planet description sheets: 154–213. Persoonia 31: 188–296. https://doi.org/10.3767/003158513X675925
- Crous PW, Wingfield MJ, Guarro J, Hernández-Restrepo M, Sutton DA, Acharya K, Barber PA, Boekhout T, Dimitrov RA, Dueñas M, Dutta AK, Gené J, Gouliamova DE, Groenewald M, Lombard L, Morozova OV, Sarkar J, Smith MT, Stchigel AM, Wiederhold NP, Alexandrova AV, Antelmi I, Armengol J, Barnes I, Cano-Lira JF, Castañeda Ruiz RF, Contu M, Courtecuisse PR, da Silveira AL, Decock CA, de Goes A, Edathodu J, Ercole E, Firmino AC, Fourie A, Fournier J, Furtado EL, Geering ADW, Gershenzon J, Giraldo A, Gramaje D, Hammerbacher A, He X-L, Haryadi D, Khemmuk W, Kovalenko AE, Krawczynski R, Laich F, Lechat C, Lopes UP, Madrid H, Malysheva EF, Marín-Felix Y, Martín MP, Mostert L, Nigro F, Pereira OL, Picillo B, Pinho DB, Popov ES, Rodas Peláez CA, Rooney-Latham S, Sandoval-Denis M, Shivas RG, Silva V, Stoilova-Disheva MM, Telleria MT, Ullah C, Unsicker SB, van der Merwe NA, Vizzini A, Wagner H-G, Wong PTW, Wood AR, Groenewald JZ (2015a) Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. http://dx.doi.org/10.3767/003158515X688433
- Crous PW, Wingfield MJ, Le Roux JJ, Richardson DM, Strasberg D, Shivas RG, Alvarado P, Edwards J, Moreno G, Sharma R, Sonawane MS, Tan YP, Altés A, Barasubiye T, Barnes CW, Blanchette RA, Boertmann D, Bogo A, Carlavilla JR, Cheewangkoon R, Daniel R, de Beer ZW, de Jesús Yáñez-Morales M, Duong TA, Fernández-Vicente J, Geering ADW, Guest DI, Held BW, Heykoop M, Hubka V, Ismail AM, Kajale SC, Khemmuk W, Kolařík M, Kurli R, Lebeuf R, Lévesque CA, Lombard L, Magista D, Manjón JL, Marincowitz S, Mohedano JM, Nováková A, Oberlies NH, Otto EC, Paguigan ND, Pascoe IG, Pérez-Butrón JL, Perrone G, Rahi P, Raja HA, Rintoul T, Sanhueza RMV, Scarlett K, Shouche YS, Shuttleworth LA, Taylor PWJ, Thorn RG, Vawdrey LL, Vidal RS, Voitk A, Wong PTW, Wood AR, Zamora JC, Groenewald JZ (2015b) Fungal Planet description sheets: 371–399. Persoonia 35: 264–327. http://dx.doi.org/10.3767/003158515X690269
- Cruse M, Telerant R, Gallagher T, Lee T, Taylor JW (2002) Cryptic species in Stachybotrys chartarum. Mycologia 94: 814–822. https://doi.org/10.1080/15572536.2003.11833175
- Desoubeaux G, García D, Bailly E, Augereau O, Bacle G, De Muret A, Bernard L, Cano-Lira JF, Garcia-Hermoso D, Chandenier J (2014) Subcutaneous phaeohyphomycosis due to Phialemoniopsis ocularis successfully treated by voriconazole. Medical Mycology Case Reports 5: 4–8. https://doi.org/10.1016/j.mmcr.2014.04.001
- Dong W, Hyde KD, Jeewon R, Doilom M, Yu XD, Wang GN, Liu NG, Hu DM, Nalumpang S, Zhang H (2021) Towards a natural classification of annulatascaceae-like taxa II: introducing five new genera and eighteen new species from freshwater. Mycosphere 12: 1–88. https://doi.org/10.5943/mycosphere/12/1/1
- Eriksson OE, Yue J-Z (1989) An amended description and disposition of the genus Thyridium. Systema Ascomycetum 8: 9–16.
- Fries EM (1823) Systema Mycologicum 2: 276–620.
- Gams W, McGinnis MR (1983) Phialemonium, a new anamorph genus intermediate between Phialophora and Acremonium. Mycologia 75: 977–987. https://doi.org/10.1080/00275514.1983.12023783
- Halleen F, Mostert L, Crous PW (2007) Pathogenicity testing of lesser-known vascular fungi of grapevines. Australasian Plant Pathology 36: 277–285. https://doi.org/10.1071/AP07019
- Hino I (1961) Icones fungorum bambusicolorum japonicorum. The Fuji Bamboo Garden, Gotenba.
- Huhndorf SM, Greif M, Mugambi GK, Miller AN (2008) Two new genera in the Magnaporthaceae, a new addition to Ceratosphaeria and two new species of Lentomitella. Mycologia 100: 940–955. https://doi.org/10.3852/08-037
- Huhndorf SM, Miller AN (2011) A molecular re-appraisal of taxa in the Sordariomycetidae and a new species of Rimaconus from New Zealand. Studies in Mycology 68: 203–210. https://doi.org/10.3114/sim.2011.68.09
- Huhndorf SM, Miller AN, Fernández FA (2004) Molecular systematics of the Sordariales: the order and the family Lasiosphaeriaceae redefined. Mycologia 96: 368–387. https://doi.org/10.1080/15572536.2005.11832982
- Hyde KD, Bao DF, Hongsanan S, Chethana KWT, Yang J, Suwannarach N (2021) Evolution of freshwater Diaporthomycetidae (Sordariomycetes) provides evidence for five new orders and six new families. Fungal Diversity 107: 71–105. https://doi.org/10.1007/s13225-021-00469-7
- Hyde KD, Chaiwan N, Norphanphoun C, Boonmee S, Camporesi E, Chethana KWT, Dayarathne MC, de Silva NI, Dissanayake AJ, Ekanayaka AH, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena R, Jiang HB, Karunarathna A, Lin CG, Liu JK, Liu NG, Lu YZ, Luo ZL, Maharachchimbura SSN, Manawasinghe IS, Pem D, Perera RH, Phukhamsakda C, Samarakoon MC, Senwanna C, Shang QJ, Tennakoon DS, Thambugala KM, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Zhang JF, Zhang SN, Bulgakov TS, Bhat DJ, Cheewangkoon R, Goh TK, Jones EBG, Kang JC, Jeewon R, Liu ZY, Lumyong S, Kuo CH, McKenzie EHC, Wen TC, Yan JY, Zhao Q (2018) Mycosphere notes 169–224. Mycosphere 9: 271–430. https://doi.org/10.5943/mycosphere/9/2/8
- Ito A, Yamada N, Kimura R, Tanaka N, Kurai J, Anzawa K, Mochizuki T, Yamamoto O (2017) Concurrent double fungal infections of the skin caused by Phialemoniopsis endophytica and Exophiala jeanselmei in a patient with microscopic polyangiitis. Acta Dermato-Venereologica 97: 1142–1144. https://doi.org/10.2340/00015555-2734
- Jaklitsch WM, Réblová M, Voglmayr H (2013) Molecular systematics of Woswasia atropurpurea gen. et sp. nov. (Sordariomycetidae), a fungicolous ascomycete with globose ascospores and holoblastic conidiogenesis. Mycologia 105: 476–485. https://doi.org/10.3852/12-244
- Khemmuk W, Geering ADW, Shivas RG (2016) Wongia gen. nov. (Papulosaceae, Sordariomycetes), a new generic name for two root-infecting fungi from Australia. IMA Fungus 7: 247–252. https://doi.org/10.5598/imafungus.2016.07.02.04
- Konta S, Hongsanan S, Liu JK, Eungwanichayapant PD, Jeewon R, Hyde KD, Maharachchikumbura SSN, Boonmee S (2017) Leptosporella (Leptosporellaceae fam. nov.) and Linocarpon and Neolinocarpon (Linocarpaceae fam. nov.) are accommodated in Chaetosphaeriales. Mycosphere 8: 1943–1974. https://doi.org/10.5943/mycosphere/8/10/16
- Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. https://doi.org/10.1093/molbev/msw054
- Leuchtmann A, Müller E (1986) Über Thyridium vestitum und sein anamorph (Ascomycetes). Botanica Helvetica 96: 283–287. http://doi.org/10.5169/seals-67205
- Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Molecular Biology and Evolution 16: 1799–1808. https://doi.org/10.1093/bioinformatics/btq224
- Luo ZL, Hyde KD, Liu JK, Bhat DJ, Bao DF, Li WL, Su HY (2018) Lignicolous freshwater fungi from China II: Novel Distoseptispora (Distoseptisporaceae) species from northwestern Yunnan Province and a suggested unified method for studying lignicolous freshwater fungi. Mycosphere 9: 444–461. https://doi.org/10.5943/mycosphere/9/3/2
- Luo ZL, Hyde KD, Liu JK, Maharachchikumbura SSN, Jeewon R, Bao DF, Bhat DJ, Lin CG, Li WL, Yang J, Liu NG, Lu Y-Z, Jayawardena RS, Li JF, Su HY (2019) Freshwater Sordariomycetes. Fungal Diversity 99: 451–660. https://doi.org/10.1007/s13225-019-00438-1
- Lutzoni F, Kauff F, Cox CJ, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lücking R, Lumbsch T, O’Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim Y-W, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. American Journal of Botany 91: 1446–1480. https://doi.org/10.3732/ajb.91.10.1446
- Mardones M, Trampe-Jaschik T, Oster S, Elliott M, Urbina H, Schmitt I, Piepenbring M (2017) Phylogeny of the order Phyllachorales (Ascomycota, Sordariomycetes): among and within order relationships based on five molecular loci. Persoonia 39: 74–90. https://doi.org/10.3767/persoonia.2017.39.04
- Martinez DA, Alberto C, Riat A, Schuhler C, Valladares P, Ninet B, Kraak B, Crous PW, Hou LW, Trellu LT (2021) Phialemoniopsis limonesiae sp. nov. causing cutaneous phaeohyphomycosis in an immunosuppressed woman. Emerging Microbes & Infections 10: 400–406. https://doi.org/10.1080/22221751.2021.1892458
- Miller AN, Huhndorf SM (2004a) A natural classification of Lasiosphaeria based on nuclear LSU rDNA sequences. Mycological Research 108: 26–34. https://doi.org/10.1017/S0953756203008864
- Miller AN, Huhndorf SM (2004b) Using phylogenetic species recognition to delimit species boundaries within Lasiosphaeria. Mycologia 96: 1106–1127. https://doi.org/10.1080/15572536.2005.11832909
- Miller AN, Huhndorf SM (2005) Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphologyin the Sordariales (Ascomycota, Fungi). Molecular Phylogenetics and Evolution 35: 60–75. https://doi.org/10.1016/j.ympev.2005.01.007
- Nitschke T (1867) Pyrenomycetes Germanici. Die Kernpilze Deutschlands. 1: 1–160. Eduard Trewendt, Breslau. https://hdl.handle.net/2027/coo.31924000644199
- Pascoe IG, Edwards J, Cunnington JH, Cottral EH (2004) Detection of the Togninia teleomorph of Phaeoacremonium aleophilum in Australia. Phytopathologia Mediterranea 43: 51–58. https://doi.org/10.1400/14571
- Perdomo H, García D, Gené J, Cano J (2013) Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia 105: 398–421. https://doi.org/10.3852/12-137
- Petch T (1917) Additions to Ceylon fungi. Annals of the Royal Botanic Gardens Peradeniya 6: 195–256. https://www.biodiversitylibrary.org/page/52423816
- Pinruan U, Sakayaroj J, Hyde KD, Jones EBG (2008) Thailandiomyces bisetulosus gen. et sp. nov. (Diaporthales, Sordariomycetidae, Sordariomycetes) and its anamorph Craspedodidymum, is described based on nuclear SSU and LSU rDNA sequences. Fungal Diversity 29: 89–98.
- Raja HA, Campbell J, Shearer CA (2003) Freshwater ascomycetes: Cyanoannulus petersenii, a new genus and species from submerged wood. Mycotaxon 88: 1–17. http://www.cybertruffle.org.uk/cyberliber/
- Rambaut A, Suchard MA, Drummond AJ (2014) Tracer 1.6. http://beast.bio.ed.ac.uk/Tracer
- Rayner RW (1970) A mycological colour chart. CMI and British Mycological Society. Kew, Surrey.
- Réblová M (2006) Molecular systematics of Ceratostomella sensu lato and morphologically similar fungi. Mycologia 98: 68–93. https://doi.org/10.1080/15572536.2006.11832714
- Réblová M (2007) Barbatosphaeria gen. et comb. nov., a new genus for Calosphaeria barbirostris. Mycologia 99: 723–732. https://doi.org/10.1080/15572536.2007.11832536
- Réblová M (2009) Teleomorph of Rhodoveronaea (Sordariomycetidae) discovered and re-evaluation of Pleurophragmium. Fungal Diversity 36: 129–139.
- Réblová M (2011) New insights into the systematics and phylogeny of the genus Jattaea and similar fungi of the Calosphaeriales. Fungal Diversity 49: 167–198. https://doi.org/10.1007/s13225-011-0099-8
- Réblová M (2013) Two taxonomic novelties in the Sordariomycetidae: Ceratolenta caudata gen. et sp nov and Platytrachelan abietis gen. et comb. nov for Ceratosphaeria abietis. Mycologia 105: 462–475. https://doi.org/10.3852/12-199
- Réblová M, Fournier J, Hyde KD (2010) Achroceratosphaeria, a new ascomycete genus in the Sordariomycetes, and re-evaluation of Ceratosphaeria incolorata. Fungal Diversity 43: 75–84. https://doi.org/10.1007/s13225-010-0032-6
- Réblová M, Fournier J, Štěpánek V (2016) Two new lineages of aquatic ascomycetes: Atractospora gen. nov. and Rubellisphaeria gen. et sp. nov., and a sexual morph of Myrmecridium montsegurinum sp. nov. Mycological Progress 15: 21. https://doi.org/10.1007/s11557-016-1166-z
- Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Studies in Mycology 68: 163–191. https://doi.org/10.3114/sim.2011.68.07
- Réblová M, Miller AN, Réblová K, Štěpánek V (2018) Phylogenetic classification and generic delineation of Calyptosphaeria gen. nov., Lentomitella, Spadicoides and Torrentispora (Sordariomycetes). Studies in Mycology 89: 1–62. https://doi.org/10.1016/j.simyco.2017.11.004
- Réblová M, Mostert L, Gams W, Crous PW (2004) New genera in the Calosphaeriales: Togniniella and its anamorph Phaeocrella, and Calosphaeriophora as anamorph of Calosphaeria. Studies in Mycology 50: 533–550.
- Réblová M, Réblová K, Štěpánek V (2015) Molecular systematics of Barbatosphaeria (Sordariomycetes): multigene phylogeny and secondary ITS structure. Persoonia 35: 21–38. http://dx.doi.org/10.3767/003158515X687434
- Réblová M, Seifert KA, Fournier J, Štěpánek V (2012) Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov and its dactylaria-like anamorph (Sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104: 1299–1314. https://doi.org/10.3852/12-035
- Réblová M, Štěpánek V (2018) Introducing the Rhamphoriaceae, fam. nov. (Sordariomycetes), two new genera, and new life histories for taxa with Phaeoisaria- and Idriella-like anamorphs. Mycologia 110: 750–770. https://doi.org/10.1080/00275514.2018.1475164
- Réblová M, Štěpánek V, Schumacher RK (2014) Xylochrysis lucida gen. et sp. nov., a new lignicolous ascomycete (Sordariomycetidae) with holoblastic conidiogenesis. Mycologia 106: 564–572. https://doi.org/10.3852/13-266
- Rehner SA, Buckley E (2005) A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. https://doi.org/10.1080/15572536.2006.11832842
- Rehner SA, Samuels GJ (1994) Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research 98: 625–634. https://doi.org/10.1016/S0953-7562(09)80409-7
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Draling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbech JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. https://doi.org/10.1093/sysbio/sys029
- Roumeguère C (1891) Fungi exsiccati praecipue Gallici. Centurie LIX. Revue Mycologique Toulouse. 13: 163–173. https://www.biodiversitylibrary.org/page/11828914
- Saccardo PA (1891) Supplementum Universale, Pars I. Agaricaceae-Laboulbeniaceae. Sylloge Fungorum 9: 1–1141. https://www.biodiversitylibrary.org/page/4300290
- Samuels GJ, Rogerson CT (1989) Endocreas lasiacidis and Sinosphaeria lasiacidis, new tropical ascomycetes. Studies in Mycology. 31: 145–149. http://www.cybertruffle.org.uk/cyberliber/
- Schoch CL, Sung G-H, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny PB, Kauff F, Wang Z, Gueidan C, Andrie RM, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson BP, Bonito G, Groenewald JZ, Arzanlou M, de Hoog GS, Crous PW, Hewitt D, Pfister DH, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh S-O, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman AY, Lumbsch TH, Lücking R, Büdel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, O’Donnell K, Sipman H, Rogers JD, Shoemaker RA, Sugiyama J, Summerbell RC, Untereiner W, Johnston PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW (2009) The Ascomycota Tree of Life: A Phylum-wide Phylogeny Clarifies the Origin and Evolution of Fundamental Reproductive and Ecological Traits. Systematic Biology 58: 224–239. https://doi.org/10.1093/sysbio/syp020
- Schwarz G (1978) Estimating the dimension of a model. The Annals of Statistics 6: 461–464. https://doi.org/10.1214/aos/1176344136
- Senanayake IC, Al-Sadi AM, Bhat JD, Camporesi E, Dissanayake AJ, Lumyong S, Maharachchikumbura SSN, Hyde KD (2018) Phomatosporales ord. nov. and Phomatosporaceae fam. nov., to accommodate Lanspora, Phomatospora and Tenuimurus, gen. nov. Mycosphere 7: 628–641. https://doi.org/10.5943/mycosphere/7/5/8
- Shenoy BD, Jeewon R, Wu WP, Bhat DJ, Hyde KD (2006) Ribosomal and RPB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycological Research 110: 916–928. https://doi.org/10.1016/j.mycres.2006.06.004
- Smith GJD, Liew ECY, Hyde KD (2006) The Xylariales: a monophyletic order containing 7 families. Fungal Diversity 13: 185–218.
- Spatafora JW, Sung GH, Johnson D, Hesse C, O’Rourke B, Serdani M, Spotts R, Lutzoni F, Hofstetter V, Miadlikowska J, Reeb V, Gueidan C, Fraker E, Lumbsch T, Lücking R, Schmitt I, Hosaka K, Aptroot A, Roux C, Miller AN, Geiser DM, Hafellner J, Hestmark G, Arnold AE, Büdel B, Rauhut A, Hewitt D, Untereiner WA, Cole MS, Scheidegger C, Schultz M, Sipman H, Schoch CL (2006) A five-gene phylogeny of Pezizomycotina. Mycologia 98: 1018–1028. https://doi.org/10.1080/15572536.2006.11832630
- Suetrong S, Klaysuban A, Sakayaroj J, Preedanon S, Ruang-Areerate P, Phongpaichit S, Pang K-L, Jones EBG (2015) Tirisporellaceae, a new family in the order Diaporthales (Sordariomycetes, Ascomycota). Cryptogamie, Mycologie 36: 319–330. https://doi.org/10.7872/crym/v36.iss3.2015.319
- Su L, Deng H, Niu Y-C (2016) Phialemoniopsis endophytica sp. nov., a new species of endophytic fungi from Luffa cylindrica in Henan, China. Mycological Progress 15: e48. https://doi.org/10.1007/s11557-016-1189-5
- Tanabe AS (2011) Kakusan4 and Aminosan: two programs for comparing nonpartitioned, proportional and separate models for combined molecular phylogenetic analyses of multilocus sequence data. Molecular Ecology Resources 11: 914–921. https://doi.org/10.1111/j.1755-0998.2011.03021.x
- Tang AMC, Jeewon R, Hyde KD (2007) Phylogenetic utility of protein (RPB2, b-tubulin) and ribosomal (LSU, SSU) gene sequences in the systematics of Sordariomycetes (Ascomycota, Fungi). Antonie van Leeuwenhoek 91: 327–349. https://doi.org/10.1007/s10482-006-9120-8
- Taylor JE, Hyde KD, Jones EBG (1997) Fungi from palms. XXXV. Thyridium chrysomallum associated with Archontophoenix alexandrae (Palmae) cultivated in Hong Kong. Sydowia 49: 94–100. https://www.zobodat.at/pdf/Sydowia_49_0094-0100.pdf
- Thongkantha S, Jeewon R, Vijaykrishna D, Lumyong S, McKenzie EHC, Hyde KD (2009) Molecular phylogeny of Magnaporthaceae (Sordariomycetes) with a new species Ophioceras chiangdaoense from Dracaena loureiroi in Thailand. Fungal Diversity 34: 157–173.
- Tsang C-C, Chan JFW, Ip PPC, Ngan AHY, Chen JHK, Lau SKP, Woo PCY (2014) Subcutaneous phaeohyphomycotic nodule due to Phialemoniopsis hongkongensis sp. nov. Journal of Clinical Microbiology 52: 3280–3289. https://doi.org/10.1128/JCM.01592-14
- Untereiner WA, Bogale M, Carter A, Hanson SÅ, Læssøe T, Štěpánek V, Réblová M (2013) Molecular phylogeny of Boliniales (Sordariomycetes) with an assessment of the systematics of Apiorhynchostoma, Endoxyla and Pseudovalsaria. Mycologia 105: 564–588. https://doi.org/10.3852/12-326
- Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. https://doi.org/10.1128/jb.172.8.4238-4246.1990
- Viljoen CD, Wingfield BD, Wingfield MJ (1999) Relatedness of Custingophora olivaceae to Gondwanamyces spp. from Protea spp. Mycological Research 103: 497–500. https://doi.org/10.1017/S0953756298007424
- Voglmayr H, Friebes G, Gardiennet A, Jaklitsch WM (2018) Barrmaelia and Entosordaria in Barrmaeliaceae (fam. nov., Xylariales) and critical notes on Anthostomella-like genera based on multigene phylogenies. Mycological Progress 17: 155–177. http://dx.doi.org/10.1007/s11557-017-1329-6
- Voigt K, Wöstemeyer J (2000) Reliable amplification of actin genes facilitates deep-level phylogeny. Microbiological Research 155: 179–195. https://doi.org/10.1016/S0944-5013(00)80031-2
- Vu D, Groenewald M, de Vries M, Gehrmann T, Stielow B, Eberhardt U, Al-Hatmi A, Groenewald JZ, Cardinali G, Houbraken J, Boekhout T, Crous PW, Robert V, Verkley GJM (2019) Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. https://doi.org/10.1016/j.simyco.2018.05.001
- White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (Eds) PCR Protocols: a guide to methods and amplifications. Academic Press, San Diego, 315–322. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
- Wong S-W, Hyde KD, Jones EBG, Moss ST (1999) Ultrastructural studies on the aquatic ascomycetes Annulatascus velatisporus and A. triseptatus sp. nov. Mycological Research 103: 561–571. https://doi.org/10.1017/S0953756298007473
- Xia JW, Ma YR, Li Z, Zhang XG (2017) Acrodictys-like wood decay fungi from southern China, with two new families Acrodictyaceae and Junewangiaceae. Scientific Reports 7: 7888. https://doi.org/10.1038/s41598-017-08318-x
- Yaguchi T, Sano A, Yarita K, Suh MK, Nishimura K, Udagawa S (2006) A new species of Cephalotheca isolated from a Korean patient. Mycotaxon 96: 309–322. http://www.cybertruffle.org.uk/cyberliber/
- Yang J, Maharachchikumbura SSN, Liu J-K, Hyde KD, Jones EBG, Al-Sadi AM, Liu Z-Y (2018) Pseudostanjehughesia aquitropica gen. et sp. nov. and Sporidesmium sensu lato species from freshwater habitats. Mycological Progress 17: 591–616. https://doi.org/10.1007/s11557-017-1339-4
- Yue J-Z, Eriksson OE (1987) Sinosphaeria bambusicola gen. et sp. nov., (Thyridiaceae fam. nov.). Systema Ascomycetum 6: 229–236.
- Zhang H, Dong W, Hyde KD, Maharachchikumbura SSN, Hongsanan S, Bhat DJ, Al-Sadi AM, Zhang D (2017) Towards a natural classification of Annulatascaceae-like taxa: introducing Atractosporales ord. nov. and six new families. Fungal Diversity 85: 75–110. https://doi.org/10.1007/s13225-017-0387-z
- Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung G-H (2006) An overview of the systematics of the Sordariomycetes based on a four-gene phylogeny. Mycologia 98: 1076–1087. https://doi.org/10.1080/15572536.2006.11832635
- Zhang N, Zhao S, Shen Q (2011) A six-gene phylogeny reveals the evolution of mode of infection in the rice blast fungus and allied species. Mycologia 103: 1267–1276. https://doi.org/10.3852/11-022