Citation: Petersen RH, Hughes KW, Justice J (2014) Two new species of Ramaria from Arkansas. MycoKeys 8: 17–29. doi: 10.3897/mycokeys.8.7356
Two species of Ramaria from the Ozark region of Arkansas, USA, R. admiratia and R. calvodistalis, are proposed as new. They are described morphologically and placed molecularly within a large clade including taxa of ramarioid and cantharelloid fungi.
Ozark Plateau, dendrophysoid hyphae, Laeticolora, taxonomy
The annual national foray of the North American Mycological Association was held in northern Arkansas in 2013, specifically in the lower Boston Mountains of the Ozark Ecoregion. Among collections of fleshy fungi were several species of Ramaria (Gomphaceae, Gomphales, Agaricomycotina), of which two appear to be new to science and are described below.
American literature dealing with the modern genus Ramaria may have begun with the treatment by
In two seminal publications,
Microscopic observations employed an Olympus BX60 microscope fitted for bright field (BF) and phase contrast (PhC) microscopy. Typical mountant was 3% aqueous KOH, with Melzer's reagent and lactic acid-Cotton blue occasionally used as stains. TFB = Tennessee Fieldbook number, assigned in the field to track notes and photos; TENN = Herbarium, University of Tennessee, permanent accession number; Q = spore length divided by spore width; Qm = mean Q value of number of spores measured; Lm = mean length of all spores measured. Colors within quotation marks are from
The term “firm-walled” is intended to describe hyphal walls which are thick enough so that hyphal crumpling or collapse is not observed in microscopic mounts, but there is no measurable distance between inner and outer wall surfaces. “Thick-walled, ” conversely, describes a hyphal wall thick enough that the inner and outer surface are sufficiently distinct and that the wall thickness can be measured and reported.
Procedures for DNA extraction, PCR and DNA sequencing of the ribosomal ITS and LSU regions was performed as described in
United States, Arkansas, Searcy Co., grounds of Shepherd of the Ozarks, 36°00'10"N, 92°28'28"W, 24.X.2013, coll. Carl Davis and Therese Martin (NAMA), TFB 14450 (TENN 69114).
admiratia = surprise, astonish, referring to the acanthodendroid hyphae in the outer stipe context.
1) Member of Ramaria subg. Laeticolora sect. Formosae; 2) clamp connections absent from all tissues; 3) acanthodendroid hyphae common in outer stipe flesh and surface; 4) stipe large, pruinose, white but easily staining to brown where handled or rubbed; 5) branch apices bright orange red; 6) type locality, northern Arkansas; 7) ITS sequence unique in the subgenus; (GenBank ITS accession KJ416133).
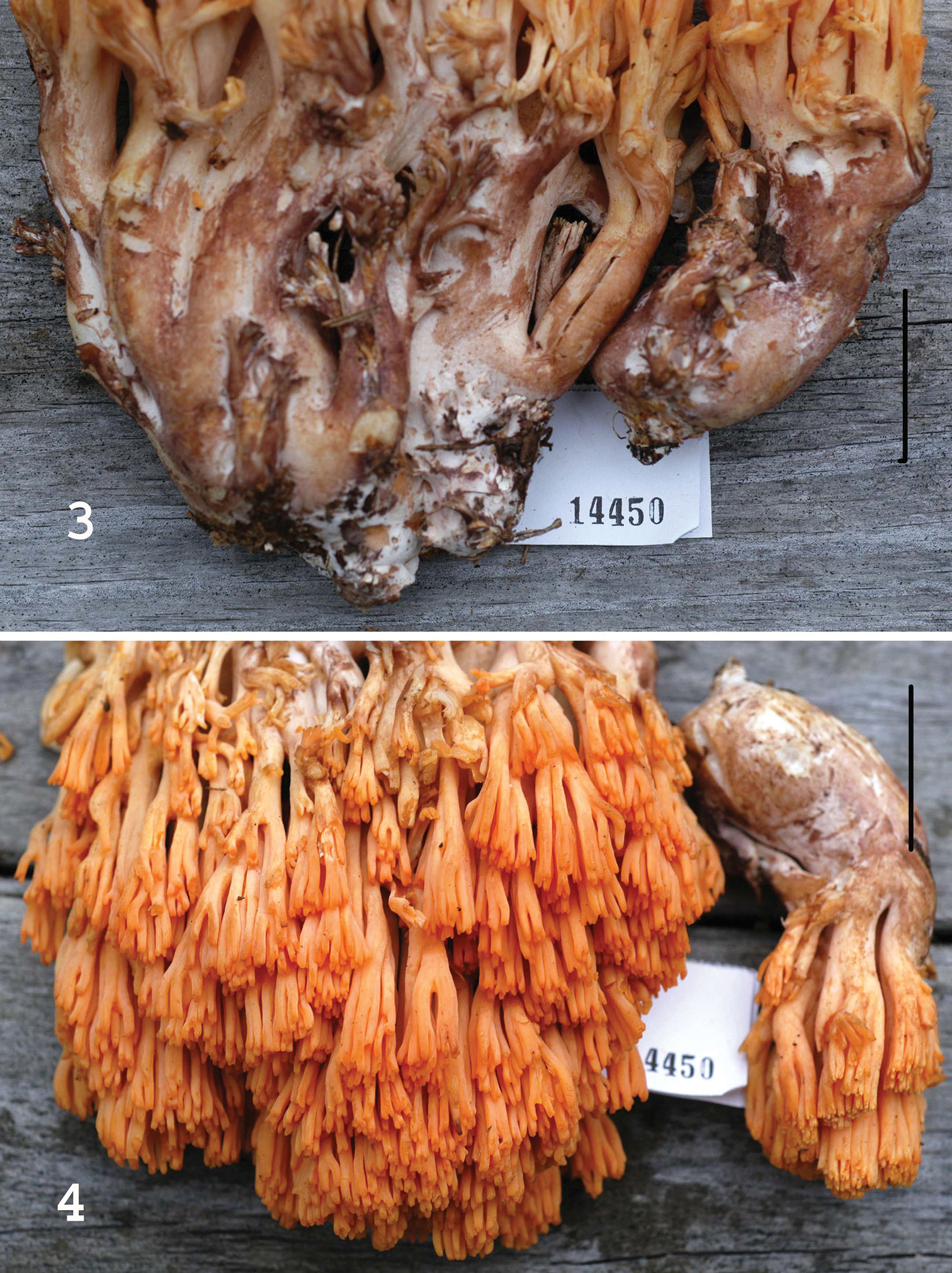
Basidiomata (Figs 1–4) robust, fleshy, –16 × 14 cm, repeatedly branched, coralloid. Stipe portion massive (Fig. 3), from discrete to 2–3 large conjunct stipes, fleshy, rounded, with minimum external mycelium, superficially white-pruinose where undisturbed, extensively “Mikado brown” (7C6) where rubbed; flesh off-white, solid, moist (not slippery), very finely marbled, very slowly becoming tan where sliced; abortive branchlets in small, vertical clusters, easily brown. Lower branches “light ochraceous buff” (5A4), upward “capucine orange” (5A6) to “Mikado orange” (6A6), in age “ochraceous salmon” (6A6) to “light ochraceous salmon” (6A4); internodes diminishing gradually; branch apices (Fig. 4) (ultimate 3–4 mm) rounded, ultimately dentate, “Grenadine red” (8A8), becoming concolorous to “capucine yellow” (5A8). Odor none. Taste none. 5% aqueous FeSO4 on stipe flesh = no color change.
Basidiomata of Ramaria admiratia. 1 Exterior of two mature basidiomata and one immature 2 Left. Exposed stipe and lower branch trama. Right. Exterior of mature basidioma. Standard lines = 5 cm. Holotype.
Basidiomata of Ramaria admiratia. 3 Stipe exteriors showing extensive brown bruising 4 Upper branches and branch apices. Standard lines = 5 cm. Holotype.
Generally second-growth hardwood forest of Quercus with scattered Carpinus, Carya and Acer; sole specimen from late autumn.
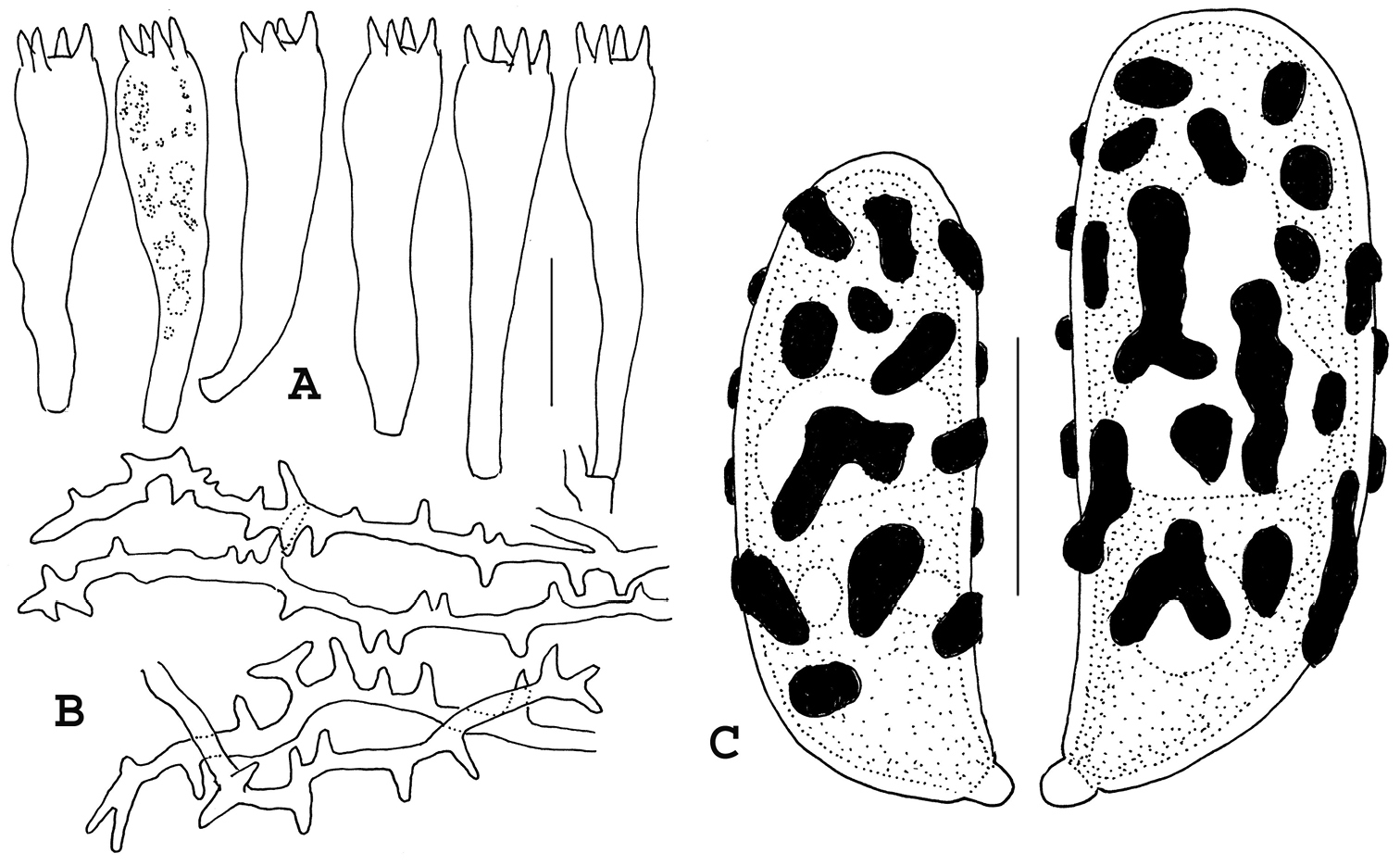
Surface of lower stipe covered with felty white tomentum; tomentum hyphae 3–4 μm diam, clampless, firm- to thick-walled (wall –0.5 μm thick), hyaline; acanthodendroid hyphae (Fig. 5B) common, refringent (PhC), strongly cyanophilous, 3–4 μm diam, thick-walled (wall –0.7 μm thick), appearing thorny. Hyphal construction of stipe medulla monomitic (with gloeoplerous hyphae), liberating significant debris in squash mounts; hyphae 4–14 μm diam, firm- to thick-walled (wall –0.5 μm thick), hyaline, clampless; ampulliform septa 7–17 μm diam, ampulliform to subspherical, thick-walled (wall –1 μm thick), delicately ornamented; gloeoplerous system represented by short lengths of cyanophilous hyphae with occasionally swellings. Hyphae of upper branch trama strictly parallel, tightly packed, thin-walled, clampless; cells filamentous to keg-shaped; occasionally slender (3–4 μm diam), non-refringent hyphae meandering through trama. Gloeoplerous system represent by occasional short lengths of strongly cyanophilous hyphae without septa. Basidia (Fig. 5A) 45–57 × 11–13 μm, clavate (not significantly bulbous), clampless, 4-sterigmate; contents multiguttulate (guttules refringent, apparently scattered throughout; PhC). Basidioles filamentous, digitate, uninformative. Basidiospores (Fig. 5B) 12–14.5 × 4.5–5.5(–6) μm (Q = 2.27–2.80; Qm = 2.52; Lm = 13.20 μm), ellipsoid, distinctly roughened in profile but ornamentation indistinct (PhC); contents heterogeneous with one or more non-refringent vacuoles (vacuoles amorphous, appearing empty on slightly darker background; PhC); ornamentation moderately cyanophilous, of narrow, axially oriented, meandering low ridges, appearing scattered, small and low in profile.
Microstructures of Ramaria admiratia. A Basidia B Acanthodendroidal hyphae of outer stipe tissue C Basidiospores showing cyanophilous ornamentation. Standard line for A, B = 20 µm; for C = 5 µm. Holotype.
Superficially, basidiomata of Ramaria admiratia resemble those of Ramaria cokeri through sordid yellow branches with reddish apices and brunnescent stipe. Ramaria cokeri belongs in Phaeoclavulina (
As noted by
The terms “monomitic, ” “dimitic”, etc. were coined by
United States, Arkansas, Baxter Co., vic. Big Flat, Rte 341, Moccasin Creek Trailhead, Ozark National Forest, 36°02'N, 92°21'W, 24.X.2013, coll. RHP, TFB 14431 (TENN 69095).
Calvus = bald; distalis = referring to the spore wall opposite the hilar appendage.
1) Member of Ramaria subg. Laeticolora; 2) clamp connections absent from all tissues; 3) acanthodendroid hyphae absent; 4) stipe small, pruinose, white, without color change where handled or rubbed; 5) branches and apices yellow; 6) type locality northern Arkansas; 7) ITS sequence unique in the subgenus; (GenBank accession KJ416132).
Adult basidiomata (Fig. 6) –15 × 12 cm, repeatedly branched, coralloid; young basidioma with discrete base, white, hardly canescent or pruinose; adult basidiome base falsely fasciculate (i.e. discrete but with narrow grooves and crevices giving the appearance of several stipes strongly compressed), snow white, finely canescent where free of soil particles; abortive branchlets common, white; stipe flesh white, solid, firm, gelatinous only in areas of degeneration or maggot-infestation, without brown bands or patches; lower branches “orange buff” (5A5), upward becoming “warm buff” (5A4) to “antimony yellow” (4B6); apices rounded, concolorous. Odor none. Taste none; consistency mealy. No bruising reactions on surface or flesh.
Basidiomata of Ramaria calvodistalis. Standard line = 5 cm. Holotype.
Possibly associated with deciduous trees from local forests of Quercus, Carya, Carpinus and occasional Pinus, solitary to gregarious, often in troops or rings; fruiting in late autumn.
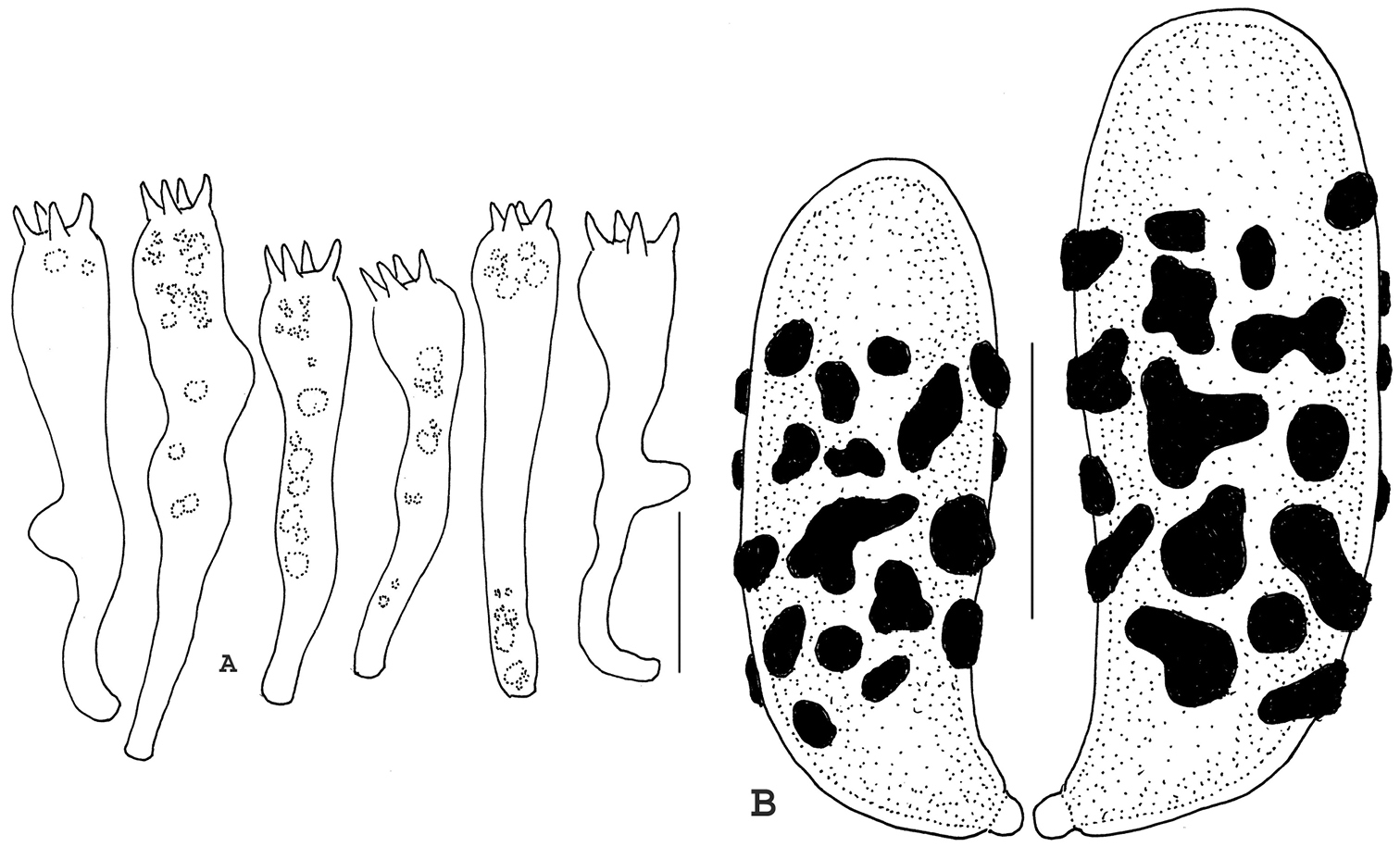
Hyphae of stipe canescence 2–4 μm diam, relatively brittle and straight, firm-walled, rarely septate, without clamp connections, non-refringent; acanthodendroid hyphae absent; in non-gelatinous areas of stipe flesh hyphae 3–12 μm diam, tortuous, frequently branched, thick-walled (wall –0.7 μm thick), often refringent (PhC), without clamp connections; rare ampulliform swellings (without clamp connection) –16 μm diam, delicately ornamented internally, not unusually thick-walled. Hyphae of upper branch trama appearing subgelatinous under low magnification, but when squashed in KOH shown to be free, 3.5–12 μm diam, without clamp connections, firm-walled (wall –0.5 μm thick); cells filamentous to elongate-barrel-shaped. Basidioles often misshapen, paraphysoid, with various small lobes or sinuate shapes. Basidia (Fig. 7A) 55–72 × 12–13 μm, clavate with somewhat bulbous apex, 4-sterigmate, occasionally with an asymmetric lobe, without clamp connections; contents usually with proximal and distal refringent guttules. Basidiospores (Fig. 7B) (12–)14–15 × 4.5–5.0(–5.5) μm (Q = 2.67–3.33; Qm = 3.03; Lm = 14.05 μm), generally boletoid, with scattered small cyanophilous warts and patches through midsection of the spore but absent from the distal end which appears bald; contents with amorphous deposits (PhC); wall slightly thickened through midsection (wall –0.5 μm thick).
Basidia and basidiospores of Ramaria calvodistalis. A Basidia B Basidiospores. Standard line for A = 20 µm; for B = 5 µm. Holotype.
Care must be taken to ascertain the condition of basidial septa. In clamped basidia, subsequent basidioles arise through the subtending clamp. In clampless basidia (as above), subsequent basidioles arise in precisely the same fashion, arising just below the subtending septum, but without the telltale evidence of a clamp connection. Additional care is required to ascertain that both tramal hyphae and basidia are without clamps. Spores are among the longest in the subgenus and largely as a result, Qm value is high. Spore outline is distinctly boletoid with slight suprahilar depression. First impression was of delicately marbled stipe flesh (i.e. with small, scattered areas of hyphae with gelatinized walls), but closer examination revealed scattered degeneration of inner stipe flesh through maggot infestation. Lower and middle branches as well as apices are essentially unicolorous, straw yellow or dull ochraceous yellow. Juvenile branches exhibit a slight blush of pale pinkish buff, but this soon fades and slowly turns to the adult yellow shades.
In the three days of the NAMA foray, numerous collections of this species were made. Basidiomata seem to occur in troops and “fairy rings” under deciduous trees and are often somewhat bulky. Stipes are not deeply rooted and are easily dislodged, but adult stipes seem consistently maggot-ridden. Because a new taxon was not anticipated, only the type collection was preserved. Although the literature dealing with Ramaria of the Pacific Northwest has been summarized at least twice over the decades (
If the key to clampless taxa in
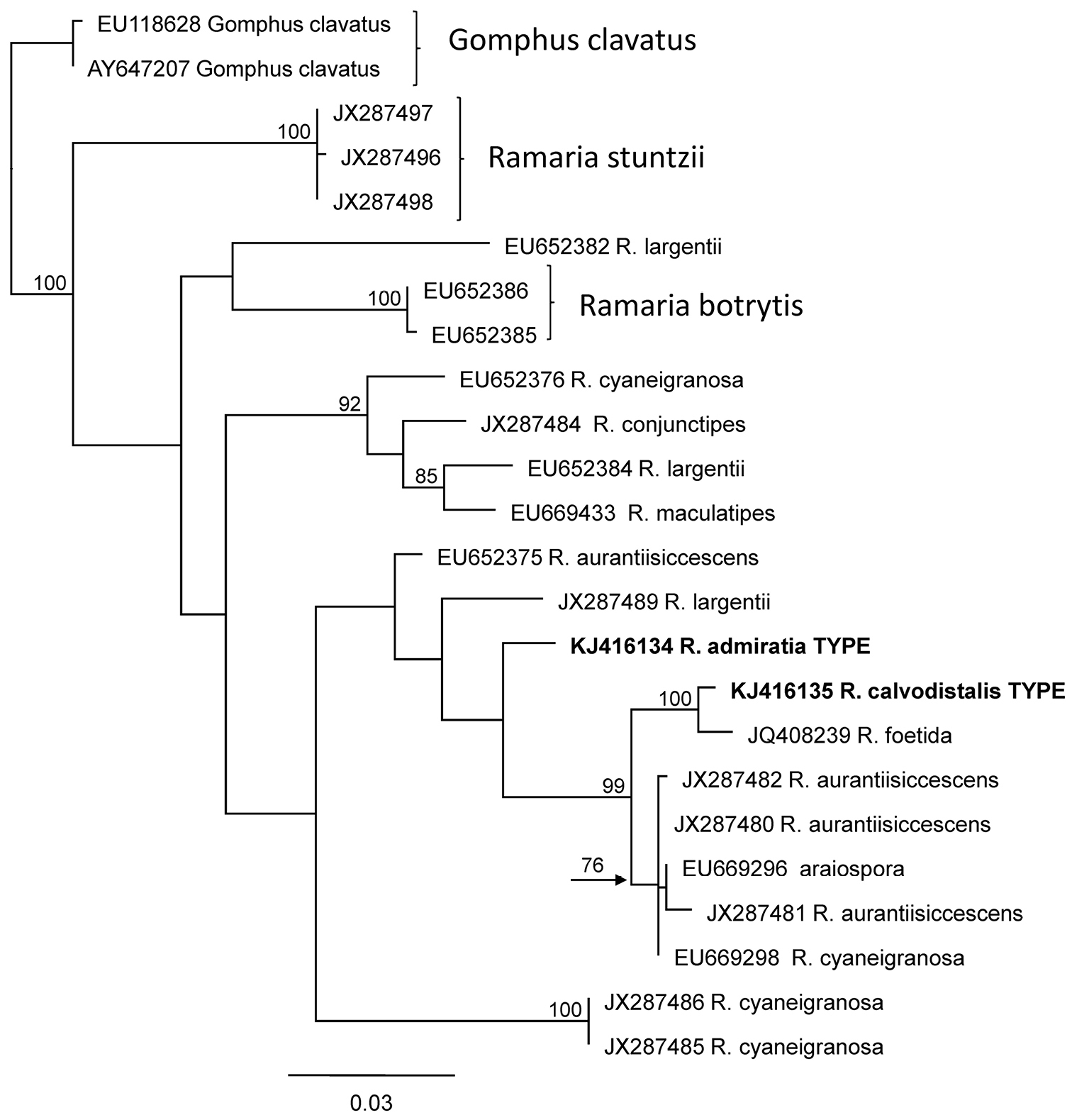
Ramaria admiratia and Ramaria calvodistalis LSU sequences place them near sequences representing brightly colored Ramaria species (Ramaria aurantiisiccescens and Ramaria araiospora) in subgenus Laeticolora (Fig. 8). ITS divergence within this subgenus is large, however, and Ramaria admiratia and Ramaria calvodistalis ITS sequences are only 86% similar to each other. Ramaria calvodistalis ITS sequences are most closely related (>97%) to two unnamed collections from Mexico (GenBank KC152173 and KC152176). These three collections differ from each other predominantly in the number of bases in repeat areas and probably represent the same lineage. We have previously noted that Mexico may have served as a glacial refugium for taxa now found further north (
PhyML Phylogeny of proposed new species of Ramaria based on nrLSU sequences. Bootstrap support equal or greater than 70% is given to the left of the supported node. GenBank accession numbers are given at the end of each twig.
Sincere thanks are extended to Carl Davis and Therese Martin of Louisiana who collected Ramaria admiratia. Work was partially supported by NSF DEB1144974 to RHP and KWH.