(C) 2013 Kare Liimatainen. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Cortinarius bovarius sp. nov., a conifer associated taxon growing on calcareous ground, is described from western North America. Phylogenetic relationships and species limits were investigated using rDNA ITS and nuclear rpb2 sequences, morphological and ecological data. The species belongs to section Bovini and its closest relative is European Cortinarius bovinus.
ITS, MrBayes, rpb2, taxonomy, Telamonia
Cortinarius is the most species rich genus of the Agaricales with a worldwide distribution. Cortinarius species are important ectomycorrhizal fungi associated with different trees and shrubs belonging e.g. to the order Fagales and families Pinaceae and Salicaceae. Lately it has also been suggested that they have a key role in the carbon cycling of boreal forests (
In recent years there have been a number of publications on the taxonomy, evolution and biogeography of species found in North America (
Material gathered by the authors from North America was studied morphologically, ecologically and sequenced to infer phylogentic relationships with other species in Bovini. DNA was extracted from dried material (a piece of lamella) with the NucleoSpin Plant kit (Macherey-Nagel, Düren, Germany). Primers ITS 1F and ITS 4 (
The combined ITS and rpb2 alignment of 11 specimens was produced with the program MUSCLE (
Bayesian inference (BI) was performed with MrBayes 3.1.2 (
Morphological descriptions are based on material collected by the authors including specimens in all stages of development. Color notations in the description follow
Specimens included in DNA analysis. Sequences produced in this study marked in bold. For acronyms of biological provinces see e.g. Knudsen and Vesterholt 2008: Funga Nordica: 32–35. * = GenBank Accession Numbers
| Species | Voucher | Herb | Locality | ITS* | rpb2* |
|---|---|---|---|---|---|
| Cortinarius bovarius (type) | 11-188 | H | U.S.A. Alaska, Fairbanks | KC905156 | KC905160 |
| Cortinarius bovarius | 11-255 | H | U.S.A. Alaska, Fairbanks | KC905158 | KC905162 |
| Cortinarius bovarius | 11-298 | H | Canada, Alberta, Hinton | KC905157 | KC905161 |
| Cortinarius bovarius | 11-373 | H | Canada, Alberta | KC905159 | KC905163 |
| Cortinarius bovinaster (type) | 04-669 | H | Finland, PeP, Ylitornio | JX407264 | JX407340 |
| Cortinarius bovinatus | 09-1520b | H | Finland, ES, Kerimäki | JX407267 | JX407341 |
| Cortinarius bovinus | 10-006 | H | Norway, Oppl, Lunner | JX407282 | JX407343 |
| Cortinarius oulankaënsis | 09-535 | H | Norway, NTr, Steinkjer | JX407295 | JX407345 |
| Cortinarius anisatus | 04-550 | H | Finland, PeP, Runteli | DQ120754 | JX407346 |
| Cortinarius neofurvolaesus | 04-001 | H | Finland, U, Helsinki | DQ139997 | JX407367 |
| Cortinarius sordidemaculatus | 04-003 | H | Finland, U, Kirkkonummi | DQ139991 | JX407368 |
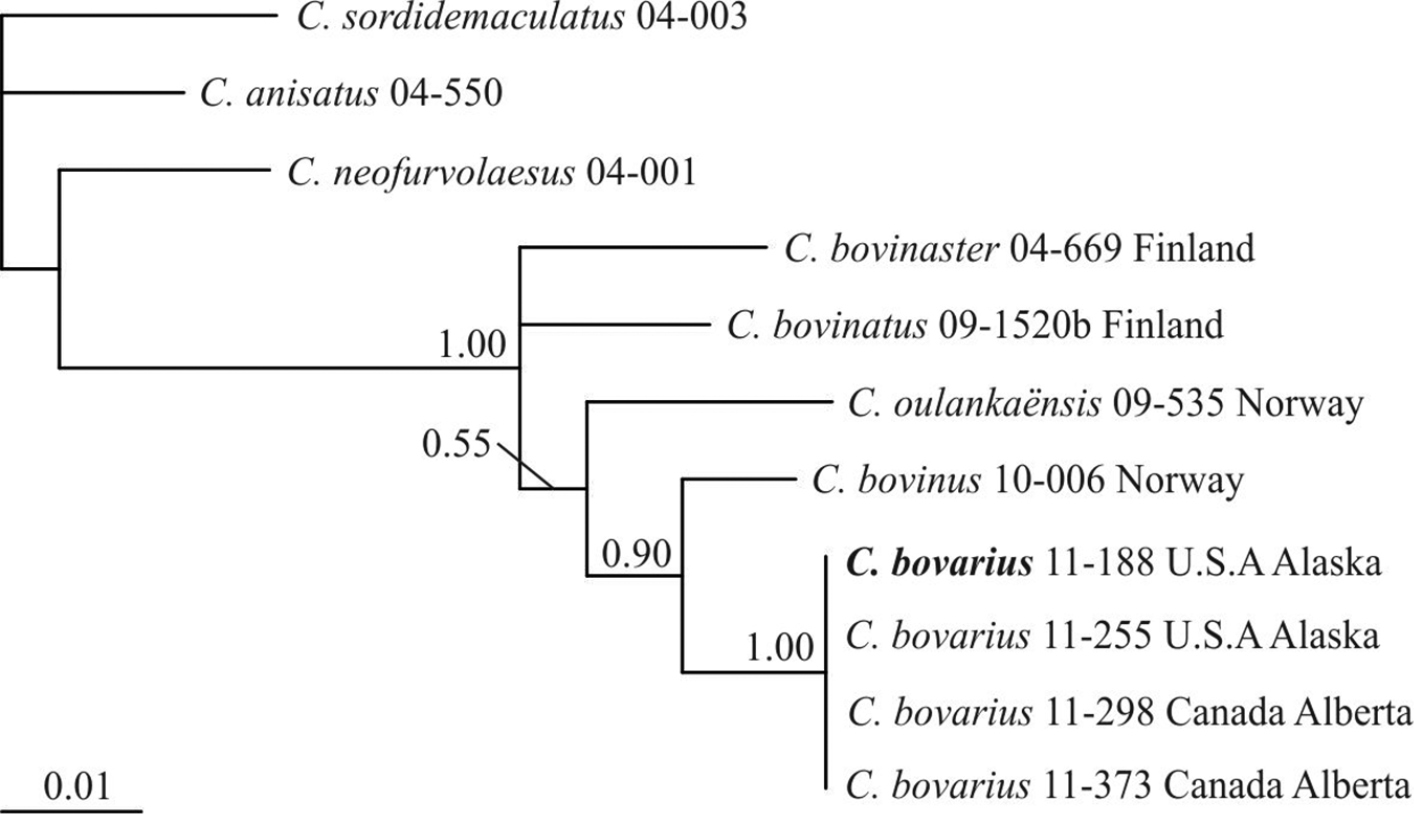
The 50% majority rule phylogram resulting from the BI analysis is shown in Fig. 1. Cortinarius bovarius is supported as a new taxon (PP 1.00). It clusters together with Cortinarius bovinus (PP 0.90) but differs from it by at least 18 substitutions and indel positions in the ITS regions and 3 substitutions in the rpb2 region. The four ITS sequences of Cortinarius bovarius have altogether 1 base and 2 length intragenomic polymorphisms. No sequences of this species exist in public databases.
The Bayesian 50% majority-rule consensus tree inferred from combined ITS and rpb2 regions. PP > 0.50 are indicated above branches.
MycoBank MB804030
http://species-id.net/wiki/Cortinarius_bovarius
Figures 2 and 3Basidiomata medium-sized to large, pileus reddish brown, smell in lamellae indistinct or slightly raphanoid. Universal veil at first white, becoming pale brown. Basidiospores 8.5–10 × 5.5–6(–6.5) μm, amygdaloid to weakly ellipsoid. In coniferous forests with Picea, on rich, calcareous ground. Belongs to sect. Bovini.
Pileus 3.5–7 cm diam., hemispherical at first, then low convex to almost plane, sometimes with a low, broad umbo, weakly fibrillose when young, later more apparent fibrillose only on the margin, somewhat waxy-glossy when moist; when young light reddish brown (5YR 6/4) to yellowish red (5YR 5/6-4/6) to reddish brown (2.5YR 5/4–4/4, 5YR 5/4–4/4), later dark red (2.5YR 3/6) to dark reddish brown (5YR 3/4–4/4, 2.5YR 3/3–3/4) and often with black spots; hygrophanous, soon drying from the center like Kuehneromyces mutabilis to lighter and more reddish brown, in dry condition reddish yellow (5YR 6/6, 7.5YR 7/6–6/6). Lamellae medium spaced to almost distant, adnexed to emarginate, fairly broad to broad, light reddish brown (5YR 6/4), light brown (7.5YR 6/3–6/4) to yellowish red (5YR 4/6), later dark reddish brown (2.5YR 3/4, 5YR 3/4–4/4), edge paler or concolorous. Stipe 5–11 cm long, 0.8–1.7 cm wide at apex, 1–3.5 cm wide at base, clavate to almost bulbose, rarely cylindrical, grayish white (silky) fibrillose, soon light reddish brown (5YR 6/3–6/4) to reddish brown (5YR 5/4) when older. Universal veil at first white, becoming pale brown, forming a girdle and thin sock-like sheath or rarely incomplete girdles on stipe surface, almost completely lost with age. Basal mycelium white. Context marbled hygrophanous, in pileus and upper part of the stipe light reddish brown (5YR 6/3–6/4) to reddish brown (5YR 4/4, 5/3), darkening towards the base of the stipe, in base reddish brown (5YR 5/3) when young, dark reddish brown (2.5YR 3/4 to 5YR 3/3–3/4) when old. Odor indistinct or slightly raphanoid. Exsiccatae: pileus brown (7.5YR 4/2–4/3) to dark brown (7.5YR 3/2–3/3), sometimes with a black center; stipe very pale brown (10YR 8/2) to light gray (10YR 7/2), in older basidiomes often darker, from grayish brown (10YR 5/2) to dark brown (10YR 4/2).
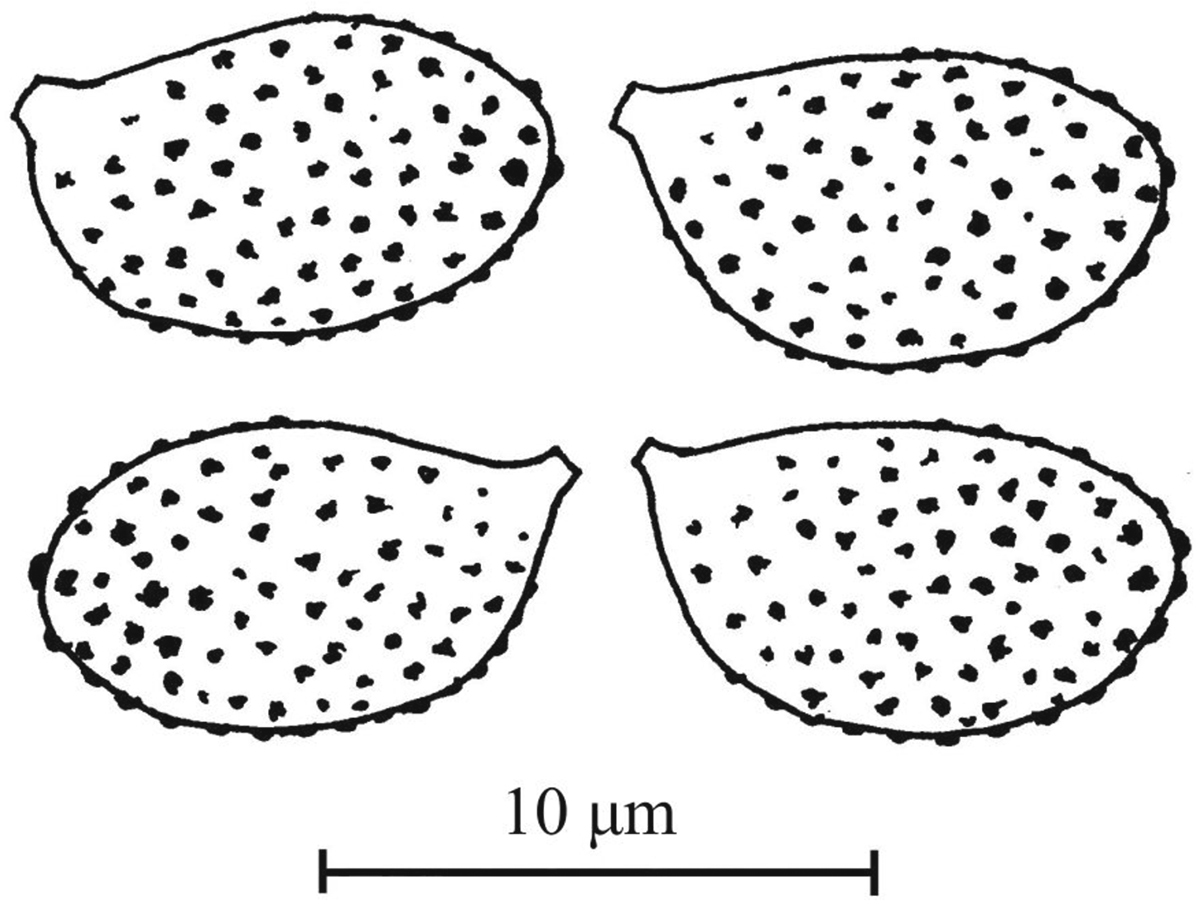
Basidiospores8.5–10 × 5.5–6(–6.5) μm, Q = 1.45–1.65, av. = 8.9–9.5 × 5.7–6.1 μm, Qav. = 1.49–1.62 (80 spores, 4 specimens, Fig. 3), amygdaloid to weakly ellipsoid, moderately verrucose, somewhat more strongly so at the apex, moderately dextrinoid. Lamellar trama hyphae smooth to very finely scabrous, sometimes with sepia colored spots. Basidia4-spored, 30–40 × 7.5–9.5 μm, almost concolorous with the background to olivaceous brownish.Pileipellis duplex, epicutis thin, hyphae 3–9 μm wide, unevenly pale brown, pigment in granules or in walls of hyphae, hypoderm distinct, elements 30–55 × 15–25(–30) μm, hyaline and smooth. Clamp connections present.
Photo of Cortinarius bovarius 11-188 (H). Photograph by K. Liimatainen.
Spores of Cortinarius bovarius 11-188 (H) in Melzer’s reagent. Drawing by T. Niskanen.
In mesic coniferous forests with Picea, on rich, calcareous soil. Known from U.S.A, Alaska and Canada, Alberta. Fruiting from late August to September.
bovarius for its affinity to Cortinarius bovinus.
U.S.A. Alaska: Fairbanks, University campus NW, trails starting from the end of Yukon road, mesic, mossy, partly needle/leaf covered Picea dominated forest with some Populus, Betula, Alnus and Salix, on rich ground, 64°51'33"N, 147°49'29"W, 22 Aug 2011, Niskanen & Liimatainen 11-188 (H, holotype; NY, isotype). GenBank no. KC905156 (ITS), KC905160 (rpb2).
Canada, Alberta, Hinton, S of center, Road to Percotte Creek, old mossy Picea dominated forest with some Populus, on calcareous ground, 53°21'53"N, 117°33'29"W, 30 Aug 2011, Liimatainen & Niskanen 11-298 (H). Alberta, Hinton, N of Athabasca river, Populus dominated forest with some Picea, 53°22'48"N, 117°51'35"W, 1040 m a.s.l., 5 Sept 2011, leg. L. Gagnon, Niskanen 11-373 (H). U.S.A. Alaska, Fairbanks, Wedgewood Resort trails, mesic Picea dominated forest with some Betula and Populus, on calcareous ground, 64°51'41"N, 147°42'46"W, 25 Aug 2011, Liimatainen & Niskanen 11-255 (H).
Cortinarius bovarius is a typical member of section Bovini, a brown species with at first a white universal veil that later becomes brownish, indistinct or slightly raphanoid smell, and occurrence on calcareous ground. It differs from its European sister species, Cortinarius bovinus, by on average narrower, less dextrinoid and less verrucose spores (those of Cortinarius bovinus on average 6.1–6.4 μm wide, fairly strongly to strongly verrucose at the apex, and fairly strongly dextrinoid). The other known species of section Bovini s. str. from western North America, Cortinarius oulankaënsis, has a more grayish brown pileus, more distant lamellae, and relatively narrower spores (Qav. = 1.61–1.65). Cortinarius bovarius is a well-defined species based on morphology and molecular data, and therefore, is here describe as new to science.
We are grateful to Martin Osis for the hospitality and all the help he provided to us during our field work in Alberta. We thank Gary Laursen for providing information on collections sites in Fairbanks. Lorraine Gagnon is thanked for collecting Cortinarius bovarius during the Alberta Mycological Society’s foray. Finally we thank both referees for their helpful comments and suggestions. This work was supported by the Academy of Finland (project # 129052).