(C) 2013 Jerry A. Cooper. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
We describe three new species, Gymnopus imbricatus, Gymnopus ceraceicola and Gymnopus hakaroa, from New Zealand that are similar to Gymnopus foetidus (= Micromphale foetidum), growing on wood, with an insititious stipe and foetid odour. The position of these species within the /gymnopoid clade is confirmed by ITS sequence analysis.
Gymnopus, Micromphale, New Zealand
The new species we describe are members of the family Omphalotaceae, Matheney et al. (2006) and have the morphological characteristics of Gymnopus section Vestipedes subsection Impudicae (Antonín & Noordeloos 2010) which contains Micromphale foetidum (Sowerby) Singer, the type species of the formerly recognised genus Micromphale (e.g. in the sense of
For this study we analysed ITS1–5.8–ITS2 data for related New Zealand collections together with representative sequences from Genbank, many from the studies cited above. The structure of our ITS tree is consistent with these previous analyses, and once again identifies a /micromphale clade closely linked to core Gymnopus species. ITS data generated for a number of representative collections of our newly described taxa support species concepts based on morphology.
Spore dimensions are stated as the mean ± 1.5 SD of 20 measurements, thus covering 86% of measurements under an assumed normal distribution model. Fresh or dried material was examined mounted in 10% KOH or Melzer’s reagent. Material was hand-sectioned. Some micrographs were obtained under DIC conditions. Measurements were always taken without DIC optics and an extended objective iris in order to maximise boundary contrast.
DNA extraction and sequencing followed the protocols outlined in
ITS Sequences used in the analysis. New sequences generated for this analysis are in bold.
| Genbank # | Collection # | Organism | PDD Voucher # | Country |
|---|---|---|---|---|
| DQ444308 | TENN50049 | Anthracophyllum archeri | New Zealand | |
| DQ480112 | TENN58672 | Gymnopus alkalivirens | Greenland | |
| DQ480114 | TENN55834 | Gymnopus alpinus | Scotland | |
| AY256691 | TENN57012 | Gymnopus aquosus | Germany | |
| DQ449971 | TENN59738 | Gymnopus aquosus | USA | |
| KC248409 | PL6304 | Gymnopus ceraceicola | PDD 101750 | New Zealand |
| KC248389 | PL126406 | Gymnopus ceraceicola | PDD 101754 | New Zealand |
| KC248400 | PL189402 | Gymnopus ceraceicola | PDD 76358 | New Zealand |
| KC248403 | JAC9334 | Gymnopus ceraceicola | PDD 80771 | New Zealand |
| KC248405 | JAC10084 | Gymnopus ceraceicola | PDD 87181 | New Zealand |
| KC248404 | JAC10336 | Gymnopus ceraceicola | PDD 87424 | New Zealand |
| KC248394 | JAC10395 | Gymnopus ceraceicola | PDD 87483 | New Zealand |
| KC248408 | JAC10817 | Gymnopus ceraceicola | PDD 87661 | New Zealand |
| KC248392 | RHP13063 | Gymnopus ceraceicola | PDD 90101 | New Zealand |
| KC248391 | KWH12891 | Gymnopus ceraceicola | PDD 90119 | New Zealand |
| KC248393 | RHP12871 | Gymnopus ceraceicola | PDD 90132 | New Zealand |
| KC248397 | JAC11005 | Gymnopus ceraceicola | PDD 95459 | New Zealand |
| KC248395 | JAC11093 | Gymnopus ceraceicola | PDD 95544 | New Zealand |
| AY256690 | TENN57012 | Gymnopus dryophilus | USA | |
| DQ449974 | TENN58087 | Gymnopus dryophilus | Costa Rica | |
| AF505778 | TENN59141 | Gymnopus dysodes | Costa Rica | |
| AY256694 | TENN59457 | Gymnopus earleae | USA | |
| DQ449973 | TFB10718 | Gymnopus exculptus | Greenland | |
| AF505780 | FB11434 | Gymnopus foetidum | USA | |
| AY256710 | TENN59217 | Gymnopus fusipes | France | |
| KC248407 | JAC9585 | Gymnopus hakaroa | PDD 81086 | New Zealand |
| KC248410 | JAC10225 | Gymnopus hakaroa | PDD 87315 | New Zealand |
| KC248411 | PL25404 | Gymnopus imbricatus | PDD 101753 | New Zealand |
| KC248406 | JAC10089 | Gymnopus imbricatus | PDD 87186 | New Zealand |
| KC248398 | JAC10310 | Gymnopus imbricatus | PDD 87398 | New Zealand |
| KC248401 | JAC10322 | Gymnopus imbricatus | PDD 87410 | New Zealand |
| KC248399 | JAC10815 | Gymnopus imbricatus | PDD 87659 | New Zealand |
| KC248402 | JAC10816 | Gymnopus imbricatus | PDD 87660 | New Zealand |
| KC248396 | JAC10495 | Gymnopus imbricatus | PDD 87675 | New Zealand |
| KC248390 | JAC11038 | Gymnopus imbricatus | PDD 95489 | New Zealand |
| AF505779 | TENN56658 | Gymnopus impudicus | Costa Rica | |
| DQ449986 | Duke RV94154 | Gymnopus iocephalus | USA | |
| AY256693 | TENN59532 | Gymnopus junquilleus | USA | |
| DQ449960 | TENN50620 | Gymnopus ocior | Switzerland | |
| DQ449972 | TENN56321 | Gymnopus subsulphureus | USA | |
| AY263453 | AWW115 | Gymnopus vitellinipes | Java/Bali | |
| AY256708 | TENN59540 | Marasmiellus juniperinus | USA | |
| GU234007 | JB14 | Marasmius androsaceus | Sweden | |
| DQ444312 | TENN50482 | Marasmius androsaceus | UK | |
| DQ444311 | TENN50704 | Marasmius androsaceus | USA | |
| DQ449990 | TENN59293 | Micromphale brassicolens | Austria |
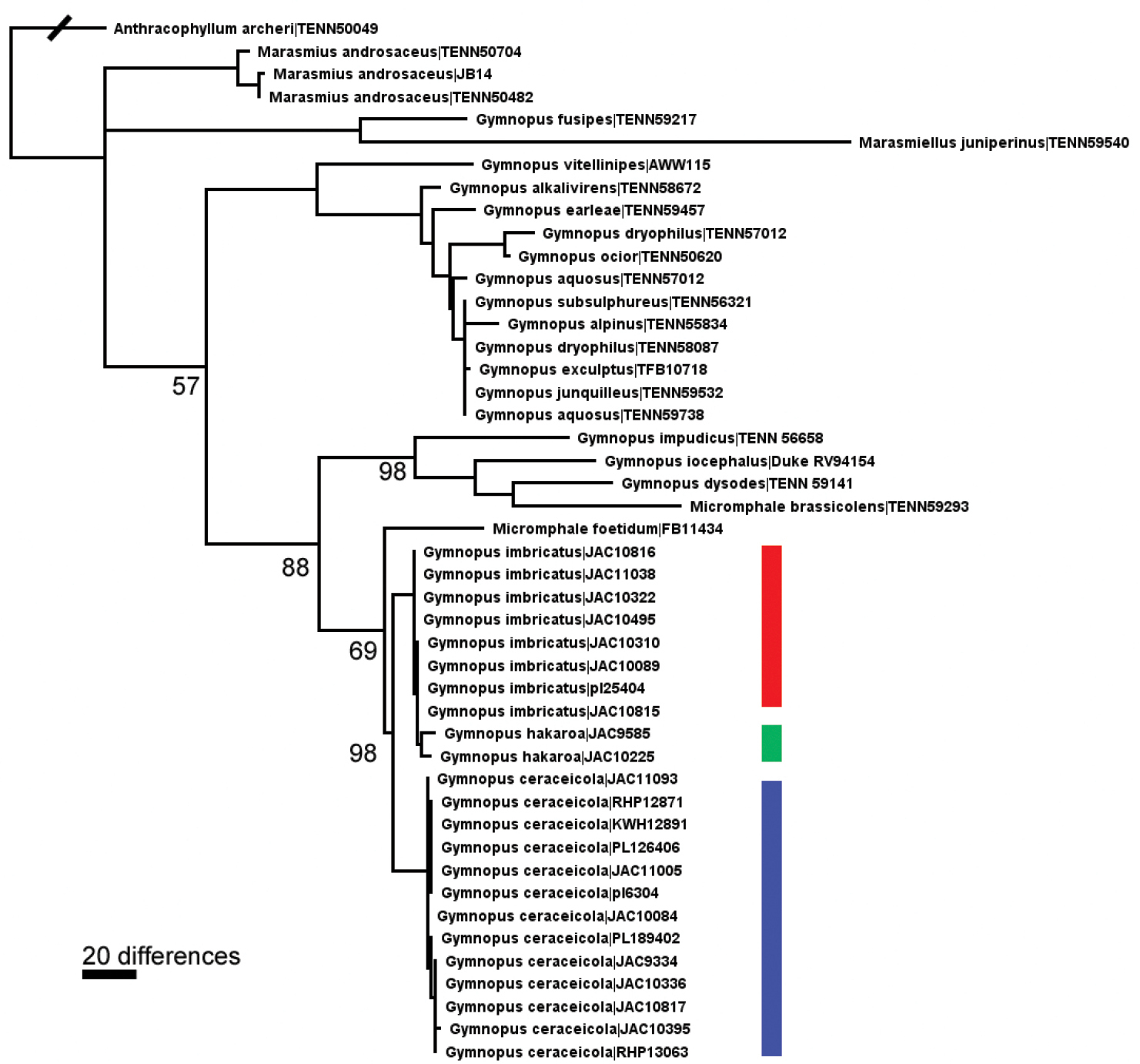
Our analysis places the New Zealand taxa in a monophyletic clade close to Gymnopus foetidum and Gymnopus brassicolens historically recognised in the genus Micromphale (Fig. 1). The combination of sequence data and morphological analysis of many collections indicate two major groups which we equate with the newly described species Gymnopus imbricatus and Gymnopus ceraceicola. In addition we recognise a further species, Gymnopus hakaroa, which is poorly distinguished from Gymnopus imbricatus on the basis of ITS sequences but which is morphologically consistently different. Minor sequence variation in the Gymnopus ceraceicola group does not correlate with morphology and we choose to recognise these specimens as a single species. More information and images of collections may be found on the Landcare Research website (Systematics Collections Data).
Maximum likelihood cladogram of selected ITS sequences, with bootstrap proportion. Red bar = Gymnopus imbricatus, green bar = Gymnopus hakaroa, blue bar = Gymnopus ceraceicola
http://species-id.net/wiki/Gymnopus_ceraceicola
Holotype: PDD 87181. Registration identifier: IF550091
Gymnopus ceraceicola is distinguished from related New Zealand species by the combination of pruinose, central stipe and dark pileus.
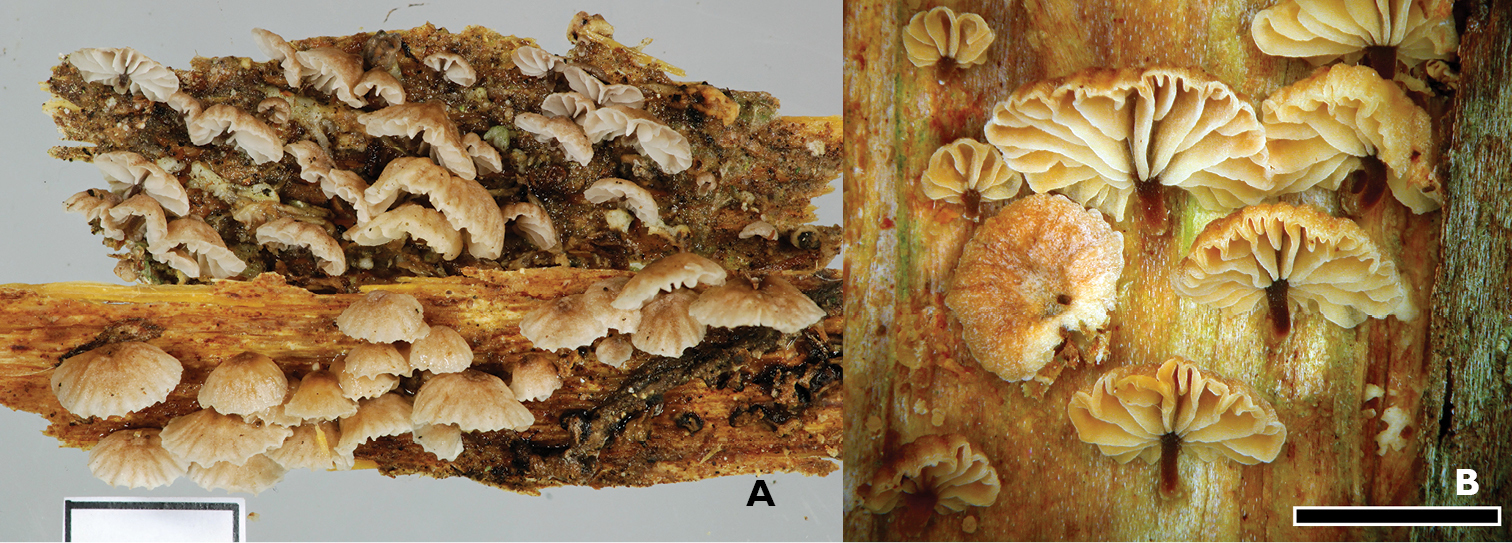
Pileus 5–20 mm, generally broadly convex to applanate, but sometimes campanulate when young, brick to purplish chestnut, minutely felty, radially furrowed and striate towards the margin, margin slightly fimbriate. Lamellae cream, creamy yellow to vinaceous buff, waxy, adnate. Lamellae present, in series of three: intercalated short/long/short. Stipe central, cartilaginous, 10–20 × 1–2 mm, equal, brown vinaceous, sometimes paler towards apex or base, always entirely finely pruinose. Stipe base insititious and always associated with a thin waxy to chalky cream layer of partially gelatinised hyphae covering the substrate. This layer is often extensive, with a distinct margin, and often green with algal cells. Fruitbodies with garlic/rotten cabbage smell, especially when crushed.
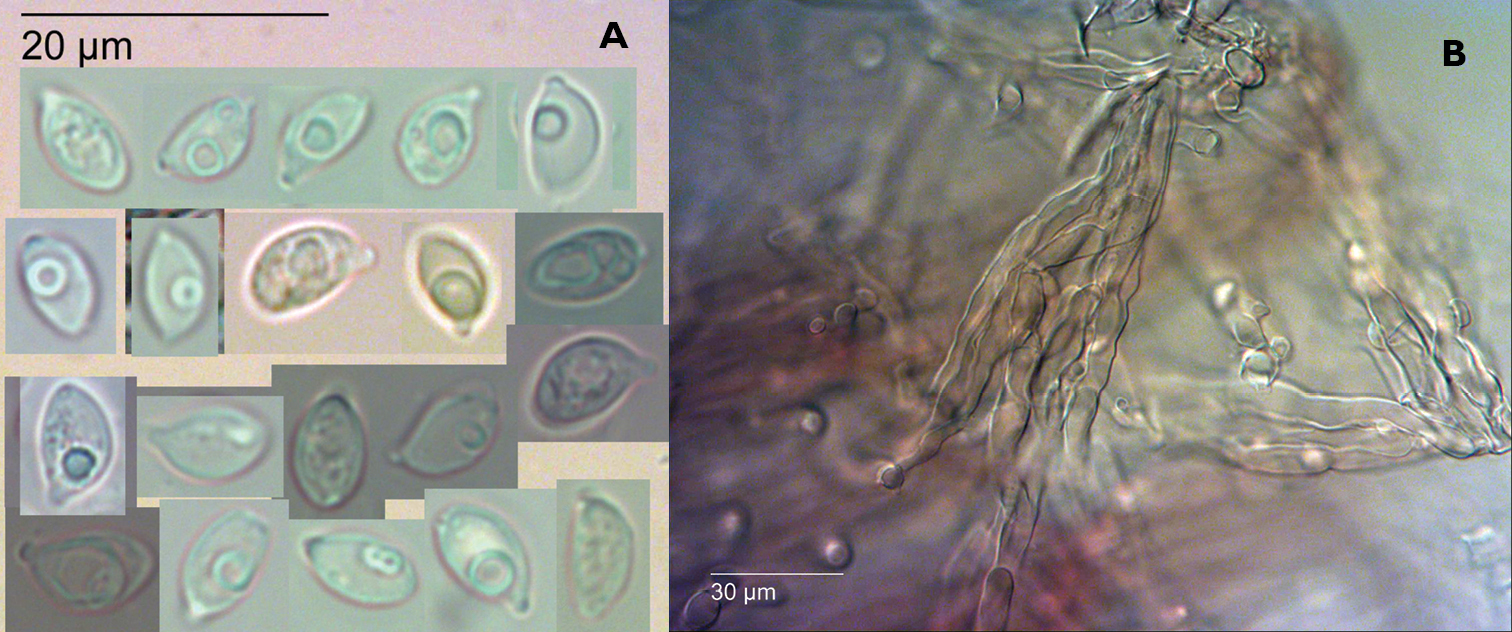
Pileipellis a partially gelatinised radially arranged clamped cutis of smooth hyphae to 5 µm diameter, with brown extra-cellular encrustation. Epidermal layer to 140 µm. Subepidermis of thick glassy–walled non-gelatinised smooth hyaline hyphae, weakly dextrinoid. Basidia clavate to 40 × 8 µm. Sterigmata to 7 µm, 4–spored. Basidioles cylindrical, tapering towards apex, 40 × 4 µm. Spores hyaline, lacrymoid, 7.9 ± 1 × 4.5 ± 0.6 µm, Q = 1.8 ± 0.1 including apiculus. Cheilocystidia and pleurocystidia not observed. Stipitipellis a cutis of brown parallel hyphae, to 5 µm wide. Caulocystidia smooth, hyaline, agglutinated into fascicles.
Colonies of a few to hundreds of fruitbodies on bark of fallen, dead branches and twigs, especially Nothofagus.
Broadly distributed and common in both North and South Islands of New Zealand.
Ceraceicola, indicating association with a basal waxy layer, although this feature is common to the three species described here.
Sequence data indicate variability in the taxon but the morphological details are constant and we choose to recognise a single species. New Zealand records of Gymnopus (Micromphale) foetidum and Gymnopus (Micromphale) brassicolens are attributable to Gymnopus ceraceicola. Authentic New Zealand material of these two species has not been identified. Gymnopus brassicolens has paler pileus colours, non-gelatinized pileipellis, cheilocystidia and pileipellis elements with lateral projections, and larger basidiospores. Gymnopus foetidus is macroscopically similar but does not possess the agglutinate fascicles of caulocystidia of Gymnopus ceraceicola.
New Zealand, North Island: PDD 40852, on dead wood, Anawhata Rd., Waitakare Ranges, Collector P.R. Johnston & G. Samuels, 9 June 1981. PDD 80771, on dead wood of Beilschmiedia tawa, Erua Forest, Taupo, Collector J.A. Cooper (JAC9334), 4 April 2005. PDD 87382, on dead wood of Nothofagus fusca, Mt Holdsworth, Gentle Annie Track, Wairarapa, Collector J.A. Cooper (JAC10294), 11 May 2007. PDD 87483, on wood, Mt Holdsworth, Donnelly Flat Loop Track, Wairarapa, Collector G. Gates & D. Ratkowsky (JAC10395), 7 May 2007. PDD 87424, on dead bark of Nothofagus, Mt Holdsworth, Gentle Annie Track, Wairarapa, Collector J.A. Cooper (JAC10336), 11 May 2007. PDD 95544, on bark of Nothofagus fusca, Rimutaka Forest Park, Wellington, Collector J.A. Cooper (JAC11093), 14 May 2009. PDD 95545, on bark of dead branch of Nothofagus fusca, Rimutaka Forest Park, Wellington, Collector J.A. Cooper (JAC11094), 14 May 2009.
New Zealand, South Island: PDD 76357, on dead twig of Nothofagus, Canaan Road Track, Nelson, Collector P.L. Leonard, 30 April 2002. PDD 96730, on dead wood, Wangapeka, Nelson, collector P.L. Leonard (PL126406), 14 April, 2006. PDD 90101=TENN 061068, on bark, vicinity of Seddonville, Charming Creek Track, Nelson, Collector R.H. Petersen (RHP 13063), 11 May 2006. PDD 76358, on bark on dead branch of Nothofagus menziesii, Lake Daniels Track, Nelson, Collector P.L. Leonard (PL189402), 2 April 2002. PDD 95459, on bark of dead branch of Nothofagus solandri, Kowai Bush, Springfield, Mid Canterbury, Collector J.A. Cooper (JAC11005), 2 May 2009. PDD 95462, on bark of dead branch of Nothofagus solandri, Kowai Bush, Springfield, Mid Canterbury, Collector J.A. Cooper (JAC11008), 2 May 2009. Holotype PDD 87181, on dead branch of Nothofagus fusca, Hinewai Reserve, Akaroa, Mid Canterbury, Collector J.A. Cooper (JAC10084), 3 June 2006. PDD 87661, on dead twigs of Leptospermum scoparium, Government Track, Waipori Falls Road, Dunedin, Collector K. Soop (JAC10817), 12 May 2008. PDD 96636, on dead wood of Nothofagus solandri, Lake Hauroko, Fiordland, Collector P. White (JAC12522). 7 May 2012. PDD 90119=TENN061007, on twigs, Vicinity of Te Anau, Kepler Track from Rainbow Reach, Fiordland, Collector K.W. Hughes (KWH12891), 30 April 2006. PDD 90132=TENN060986, vicinity Manapouri, Borland Lodge, Nature Track, Fiordland, Collector R.H. Petersen (RHP12871), 29 April 2006.
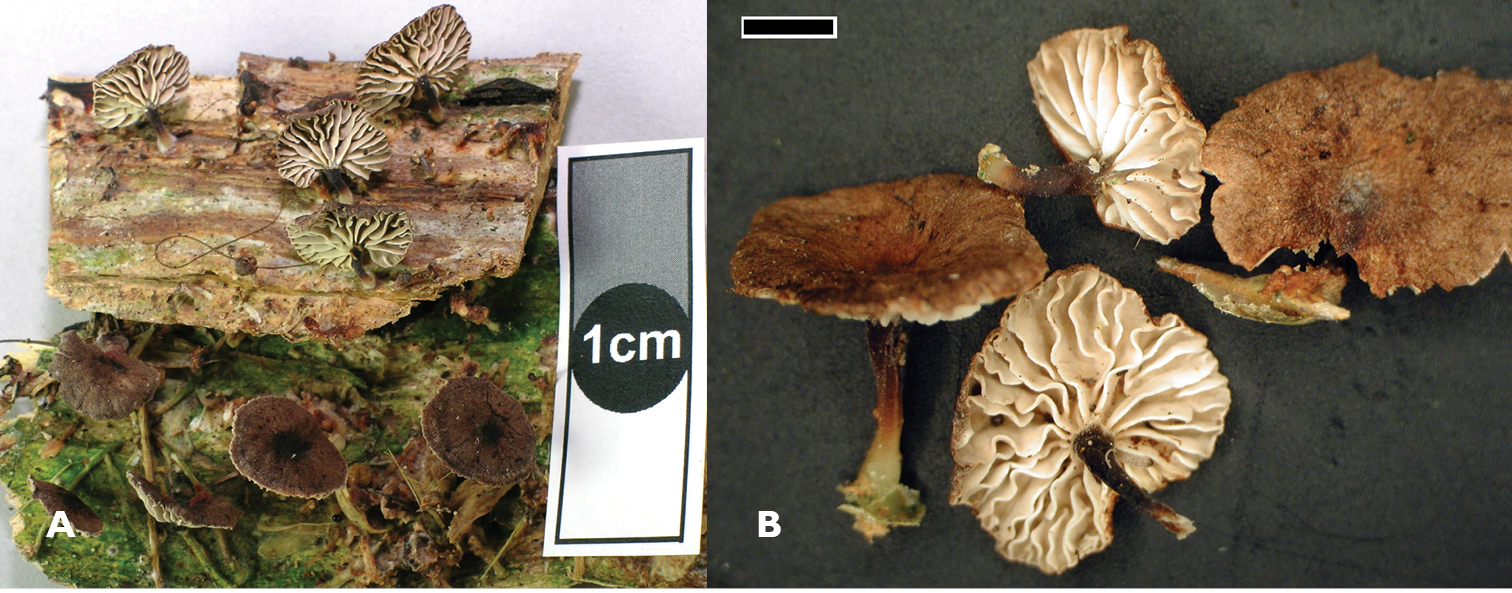
Gymnopus ceraceicola Holotype, PDD 87181. Fruitbodies.
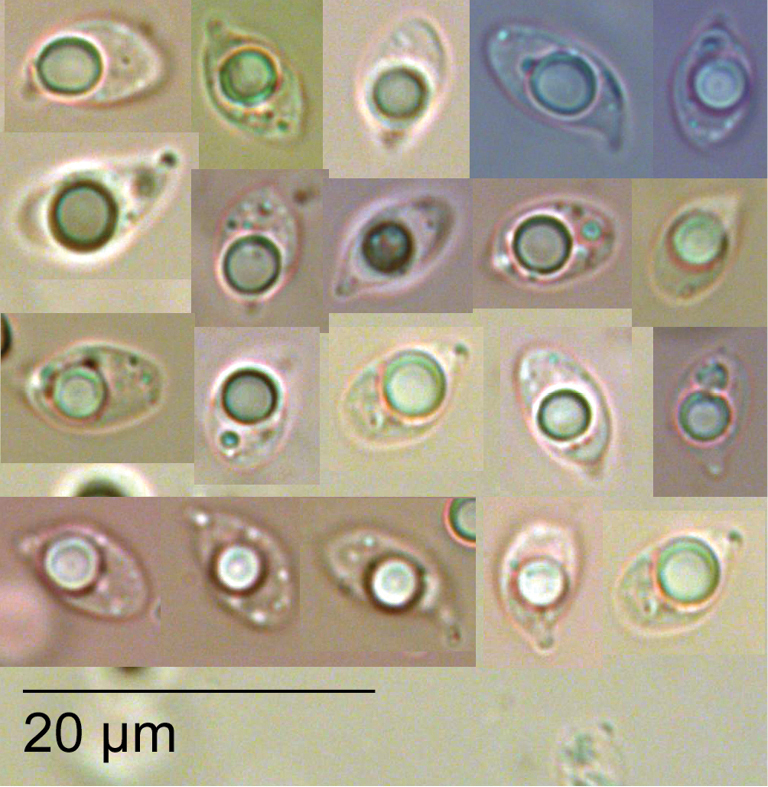
Gymnopus ceraceicola Holotype, PDD 87181. A Spores (KOH) B Agglutinated fascicles of caulocystidia on stipe (KOH).
http://species-id.net/wiki/Gymnopus_imbricatus
Holotype: PDD 95489. Registration identifier: IF550092
Gymnopus imbricatus is distinguished from related New Zealand species by the smooth stipe, larger basidiospores, and imbricate habit.
Pileus 3–20 mm in diameter convex, cream to fawn, minutely felty, radially furrowed and striate towards the margin, margin fimbriate. Lamellae cream to creamy yellow, adnate. Lamellae present, in series of two: short/long. Stipe mostly eccentric, cartilaginous, to 3 × 0.5 mm, equal, umber to black, sometimes paler towards base, always entirely smooth. Stipe base insititious and usually associated with a thin waxy to chalky cream layer of partially gelatinised hyphae covering the substrate, usually green with algal cells. Fruitbodies with garlic/rotten cabbage smell, especially when crushed.
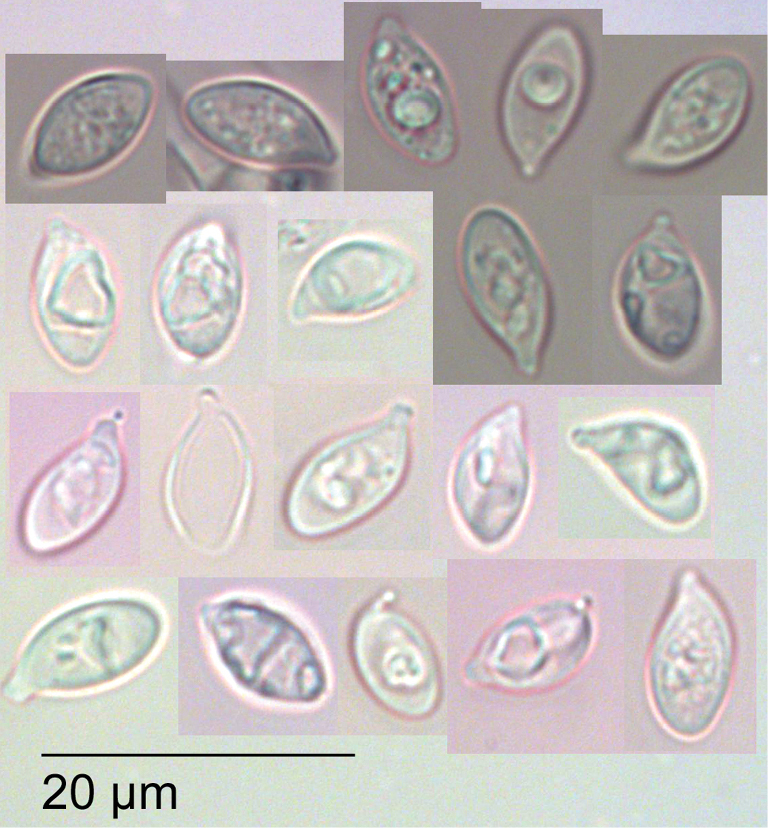
Pileipellis a partially gelatinised irregular clamped cutis of hyphae 4 µm diameter, without intra or extracellular pigmentation, terminal layer with gelatinised coralloid elements, to 2 µm wide, and occasional small finger-like trichodermal elements to 20 µm. Epidermal layer to 25 µm. Subepidermis of thick glassy-walled non-gelatinised smooth hyaline hyphae, weakly dextrinoid. Basidia clavate to 50 × 10 µm. Sterigmata to 5 µm, 4–spored. Basidioles to 50 × 6 µm cylindrical and tapered towards apex. Spores hyaline, lacrymoid 9.8 ± 1.2 × 5.1 ± 0.4 µm, Q = 1.9 ± 0.3 including apiculus. Cheilocystidia and pleurocystidia not observed. Stipitipellis a cutis of parallel brown hyphae, to 6 µm wide. Caulocystidia absent.
Forming imbricate colonies of dozens to hundreds of fruitbodies on bark and decorticate wood of dead branches and twigs, especially Kunzea and Leptospermum but occurs with other trees. Also occurs at the stem base of live trees.
Broadly distributed and common in both North and South Islands of New Zealand.
Imbricatus, pertaining to the often tiered and overlapping eccentrically stemmed caps.
New Zealand, North Island: PDD 80766, on bark of Beilschmedia tawa, Erua Forest, Taupo, collector J.A. Cooper (JAC9329), 4 April, 2005. PDD 87398, bark on dead branch of Nothofagus, Waiohine Gorge, Wairarapa, Collector J.A. Cooper (JAC10310), 10 May 2007. PDD 87410, dead stems of Ripogonum scandens, Waiohine Gorge, Wairarapa, Collector J.A. Cooper (JAC10322), 10 May 2007.
New Zealand, South Island: PDD 101753, dead branches of Nothofagus menziesii, Riwaka Resurgence, Nelson, Collector P.L. Leonard (PL25404), 10 April, 2006. PDD 96141, dead twigs of Kunzea ericoides, Mt Fyffe Track, Kaikoura, collector J.A. Cooper (JAC11734), 26 Feb. 2011. PDD 80154, dead log of Nothofagus menziesii, Lewis Pass, Buller, collector J.A. Cooper (JAC8287), 24 November, 2001. PDD 80157, on dead de-corticate log, Lyell Walkway, Nelson, collector J.A. Cooper (JAC80157), 25 November, 2001. PDD 87675, living stem of Fuchsia excorticata, Saddle Hill, Mid Canterbury, Collector J.A. Cooper (JAC10495), 22 May 2005. Holotype PDD 95489 (Figs 4 and 5), base of live trees of Kunzea ericoides, Kennedy’s Bush, Mid Canterbury, Collector J.A. Cooper (JAC11038), 24 May 2009. PDD 79799, bark of dead tree, Kennedy’s Bush, Mid Canterbury, Collector J.A. Cooper (JAC8921), 20 March 2004. PDD 87186, on bark of living tree of Kunzea ericoides, Hinewai Reserve, Akaroa, Mid Canterbury, Collector J.A. Cooper (JAC10089), 3 June 2006. PDD 87660, fallen log, Racemans Track, Silverstream Valley, Dunedin, Collector S. Dodd (JAC10816), 13 May 2008. PDD 87659, on dead twigs of Kunzea ericoides, Evansdale Glen, Dunedin, Collector P.R. Johnston (JAC10815), 12 May 2008.
Gymnopus imbricatus. A Holotype PDD 95489. Fruitbodies, scale 1 cm B PDD 87186. Scale 1 mm.
Gymnopus imbricatus Holotype PDD 95489. Spores (in KOH).
http://species-id.net/wiki/Gymnopus_hakaroa
Holotype: PDD 87315. Registration identifier: IF550093
Gymnopus hakaroa is distinguished from Gymnopus ceraceicola by smaller stature and a pruinose stipe lacking fascicles of agglutinate caulocystidia. It is distinguished from Gymnopus imbricatus by non-imbricate growth, a consistently central stipe, and smaller basidiospores.
Pileus 3–10 mm diam. convex, rusty tawny to umber, minutely felty, weakly radially furrowed and striate towards the margin. Lamella cream to yellow, waxy. Lamellae present, in series of three: intercalated short/long/short. Stipe central, cartilaginous, to 5 × 0.6 mm, equal, umber to black, paler towards base, smooth to minutely pruinose. Stipe base insititious and always associated with an obvious waxy to chalky cream layer of partially gelatinised hyphae covering the substrate, usually green with algal cells. Fruitbodies with garlic/rotten cabbage smell, especially when crushed.
Pileipellis a partially gelatinised radially arranged clamped cutis of smooth hyphae to 3 µm in diameter, with brown extra-cellular encrustation. Epidermal layer to 80 µm. Subepidermis of thick glassy-walled non-gelatinised smooth hyaline hyphae, to 3 µm in diameter, weakly dextrinoid. Basidia clavate to 40 × 8 µm. Sterigmata to 7 µm, 2–4-spored. Basidioles cylindrical and tapered towards apex 40 × 6 µm. Spores hyaline, lacrymoid 8.3 ± 1 × 4.8 ± 0.3 µm, including apiculus, Q = 1.7 ± 0.2. Cheilocystidia and pleurocystidia not observed. Stipitipellis a cutis of hyaline to pale brown hyphae, to 5 µm wide. Stipe without caulocystidia.
Forming imbricate colonies of dozens to hundreds of fruitbodies on decorticate dead wood.
Currently Gymnopus hakaroa is only known from a single location on the Canterbury Port Hills in the South Island of New Zealand.
Hakaroa, a Maori name for the Bank’s Peninsula region of New Zealand.
Sequence data (Fig. 1) indicates a close phylogenetic relationship to Gymnopus imbricatus but there are consistent and substantial morphological differences.
New Zealand, South Island: Holotype PDD 87315 (Figs 6 and 7) on dead log, Kennedys Bush Reserve, Port Hills, Mid Canterbury, Collector J.A. Cooper (JAC10225), 11 Feb. 2007. PDD 81086 (Fig. 8), on dead wood of Kunzea ericoides, Kennedys Bush Reserve, Port Hills, Mid Canterbury, Collector J.A. Cooper (JAC9585), 23 July, 2007. PDD 96390, on dead decorticate log of Melicytus ramiflorus, Kennedys Bush Reserve, Port Hills, Mid Canterbury, Collector J.A. Cooper (JAC11301), 17 April, 2010.
Gymnopus hakaroa A PDD 81086. Fruitbodies B scale= 2 mm
Gymnopus hakaroa Holotype PDD 87315. Fruitbodies, showing waxy substratum.
Gymnopus hakaroa Holotype PDD 87315. Spores (KOH).
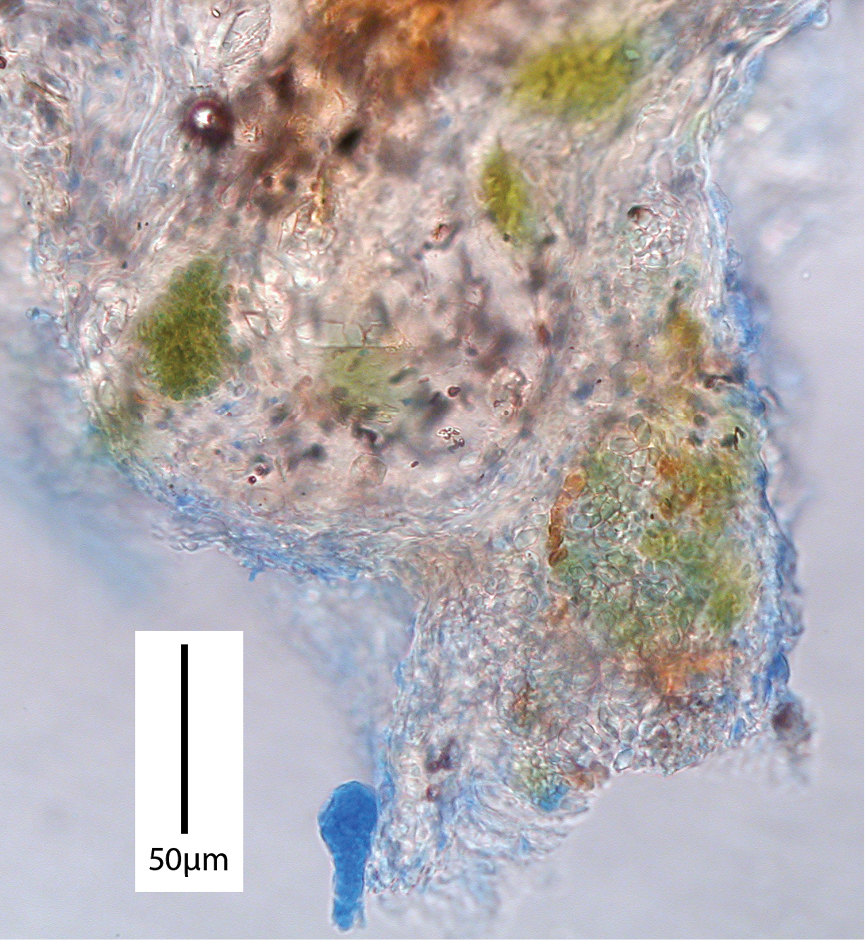
Gymnopus hakaroa PDD 96390. Stipe base (arrow) with surrounding algal mat. Inset, primordial arising from algal mat.
Gymnopus hakaroa PDD 96390. Pockets of algal cells embedded in hyphal tissue of stem base (cotton blue stain).
Gymnopus imbricatus, as its name suggests forms dense populations of small imbricate fruitbodies. It is most commonly associated with tea-tree (Kunzea ericoides and Leptospermum scoparium) and often found on the bark at the base of living trees. Gymnopus hakaroa is larger, with a dark minutely pruinose cap and again forms dense populations on the bark of dead logs. These two species have smooth stems. Gymnopus ceraceicola is distinguished by larger fruitbodies, a pruinose stipe, and is more commonly associated with southern-beech forests on dead fallen logs. The species of Gymnopus described here belong in the /micromphale clade of
Thanks to Dukchul Park, Landcare Research, for DNA extraction and sequencing, and to Sapphire McMullen-Fisher for material of Marasmiellus affixus. The New Zealand Department of Conservation is thanked for permission to collect specimens from reserves and national parks that they manage. The first author was supported through the Landcare Research Systematics Portfolio, with Core funding support from the Science and Innovation Group of the New Zealand Ministry of Business, Innovation and Employment.