(C) 2013 A. Martyn Ainsworth. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Citation: Ainsworth AM, Cannon PF, Dentinger BTM (2013) DNA barcoding and morphological studies reveal two new species of waxcap mushrooms (Hygrophoraceae) in Britain. MycoKeys 7: 45–62. doi: 10.3897/mycokeys.7.5860
Rigorous diagnostics and documentation of fungal species are fundamental to their conservation. During the course of a species-level study of UK waxcap (Hygrophoraceae) diversity, two previously unrecognized species were discovered. We describe Gliophorus europerplexus sp. nov. and G. reginae sp. nov., respectively orange–brown and purple–pink waxcap mushrooms, from nutrient-poor grasslands in Britain. Both share some morphological features with specimens assigned to Gliophorus (=Hygrocybe) psittacinus. However, analysis of sequences of the nuclear ITS DNA barcode region from these and related taxa confirms the phylogenetic distinctness of these lineages. Furthermore, we demonstrated that the holotype of Hygrophorus perplexus, a North American species morphologically resembling G. europerplexus, is phylogenetically divergent from all our collections. It is likely that further collections of G. europerplexus will be revealed by sequencing European material currently filed under G. perplexus and its synonyms. However, two such collections in the Kew fungarium yielded sequences that clustered together but were divergent from those of G. europerplexus, G. perplexus and G. psittacinus and may represent a further novel taxon. By contrast, G. reginae is morphologically distinct and can usually be recognized in the field by its purplish viscid pileus and relatively stout, flexuose, pale stipe. It is named to commemorate the diamond jubilee of Her Majesty Queen Elizabeth II in 2012 and the 60th anniversary of her coronation in 2013.
Conservation, cryptic species, diamond jubilee, DNA barcoding, fungi, Gliophorus, Glutinosae, Hygrophoraceae, parrot waxcap, taxonomy, waxcap grasslands
In Europe, waxcap mushrooms (Hygrophoraceae, Hygrocybe s.l.) are conspicuous and often attractive features of nutrient-poor short turf. Hotspots of waxcap taxonomic diversity are usually biocide-free, unfertilised or semi-improved, grazed grasslands or mown lawns with low levels of soil disturbance and long periods of ecological continuity. Such sites are often, but not always, of low botanical interest and this has undoubtedly delayed their recognition as sites of international conservation importance. Historically, therefore, even the best “waxcap grasslands” (using
Waxcaps are regarded as nitrogen-sensitive organisms because fruiting is inhibited by applications of nitrogenous fertilizers (
Taxonomic treatments of European waxcaps have recognized from one to seven genera (e.g.
Waxcap identification in Britain and Ireland currently adheres to
Recent developments in DNA-based methods of identification (“DNA barcoding”) are revolutionizing rapid diagnosis of diversity in mushrooms and other Fungi (
This paper focuses on our treatment of two unusual waxcaps that, because of their viscid pilei and subregular hymenophoral tramal hyphae, are assigned to the segregate genus Gliophorus. They share some morphological characters with the parrot waxcap Gliophorus psittacinus, which encompasses a wide range of basidiomatal pigmentation based on current concepts. Numerous colour forms can be recognised (
A total of 20 collections corresponding to the Gliophorus psittacinus complex were sequenced and morphologically examined in the current study. These comprised 12 recent UK field collections now in K, four existing K collections from UK and Jersey and four US type collections in MICH. Table 1 shows the relevant collection details. Further details for specimens of Gliophorus europerplexus and Gliophorus reginae are provided in the taxonomic treatment below. Geographical coordinates of collections were converted from Ordnance Survey National Grid References, based on the OSGB36 datum, to latitude and longitude (WGS84 datum).
Collection and voucher information for the specimens used in this study.
| GenBank Accession No. |
Taxon | Fungarium Accession No. |
Collection code/ seq. literature ref. | Source | Notes |
|---|---|---|---|---|---|
| KF218268 | Gliophorus europerplexus | K(M)181241 | E.J.M.Arnolds WX359 | UK, Wales, Merionethshire | |
| KF218266 | Gliophorus europerplexus | K(M)181245 | D.J.Harries DJH064A WX663 | UK, Wales, Pembrokeshire | |
| KF218267 | Gliophorus europerplexus | K(M)181246 | D.J.Harries DJH064B WX664 | UK, Wales, Pembrokeshire | Holotype |
| KF218272 | Gliophorus perplexus | MICH10924 | A.H.Smith 21491 | USA, Michigan, Cheboygan Co. | Hygrophorus perplexus holotype, part |
| KF218270 | Gliophorus perplexus | MICH45363 | T.E.Brooks 1098 | USA, Michigan, Cheboygan Co. | Hygrophorus perplexus paratype |
| KF218271 | Gliophorus perplexus | MICH45364 | T.E.Brooks 1099 | USA, Michigan, Cheboygan Co. | Hygrophorus perplexus paratype |
| KF218274 | Gliophorus perplexus aff. | K(M)121495 | E.W.Brown | Channel Islands, Jersey | as Hygrocybe psittacina var. perplexa |
| KF218273 | Gliophorus perplexus aff. | K(M)166625 | N.W.Legon | UK, England, South Somerset | as Hygrocybe psittacina var. perplexa |
| KF218269 | Gliophorus perplexus | MICH45365 | A.H.Smith 34029 | USA, Michigan, Chippewa Co. | Hygrophorus perplexus paratype |
| EU784339 | Gliophorus psittacinus | K(M)127070 | UK, N. Ireland, Derry | ||
| EU784340 | Gliophorus psittacinus | K(M)127194 | UK, N. Ireland, Tyrone | ||
| EU784341 | Gliophorus psittacinus | K(M)90029 | UK, England, Buckinghamshire | ||
| EU784342 | Gliophorus psittacinus | K(M)90674 | UK, England, East Sussex | ||
| FM208875 | Gliophorus psittacinus | Hungary, Kétvölgy | |||
| FM208895 | Gliophorus psittacinus | Hungary, Apátistvánfalva | |||
| KF218259 | Gliophorus reginae | K(M)156265 | R.Winnall | UK, England, Worcestershire | Holotype |
| KF218258 | Gliophorus reginae | K(M)181115 | R.D.Foster WCS15 WX115 | UK, England, Derbyshire | |
| KF218260 | Gliophorus reginae | K(M)181116 | R.D.Foster WCS26 WX126 | UK, England, Derbyshire | |
| KF218263 | Gliophorus reginae | K(M)181117 | R.Winnall WX459 | UK, England, Worcestershire | |
| KF218265 | Gliophorus reginae | K(M)181124 | J.E.Hodges DJH055 WX535 | UK, Wales, Pembrokeshire | |
| KF218264 | Gliophorus reginae | K(M)181126 | R.Winnall WX673 | UK, England, Worcestershire | |
| KF218275 | Gliophorus reginae | K(M)181127 | R.Winnall & A.M.Ainsworth WX694 | UK, England, Worcestershire | |
| KF218257 | Gliophorus reginae | K(M)181128 | R.Winnall & A.M.Ainsworth WX695 | UK, England, Worcestershire | |
| KF245883 | Gliophorus reginae | K(M)181129 | R.Winnall & A.M.Ainsworth WX696 | UK, England, Worcestershire | |
| KF218262 | Gliophorus reginae | K(M)181227 | R.Winnall WX461 | UK, England, Worcestershire | |
| KF218261 | Gliophorus reginae | K(M)41524 | C.Lovatt | UK, England, Staffordshire |
Spore measurements are rounded to the nearest half micron and preceded by associated data in square brackets. For example, [60, K(M)181128*, K(M)181129] would indicate that 60 spores in total were measured either in water from prints (Gliophorus reginae) or in Melzer’s reagent from lamellar squashes (Gliophorus europerplexus) from the collections K(M)181128 and K(M)181129. Collections sequenced during this study, such as K(M)181128 in this example, are denoted throughout by *. Measurements of basidia and other hyphal elements are rounded to the nearest micron. Colours given in parentheses refer to those shown in a standard mycological identification chart (
DNA was extracted using either an enzymatic digestion-glass fiber filtration protocol in 96-well plate format with a vacuum-manifold or the Whatman FTA® card method described in
Six additional sequences labelled as Hygrocybe psittacina (
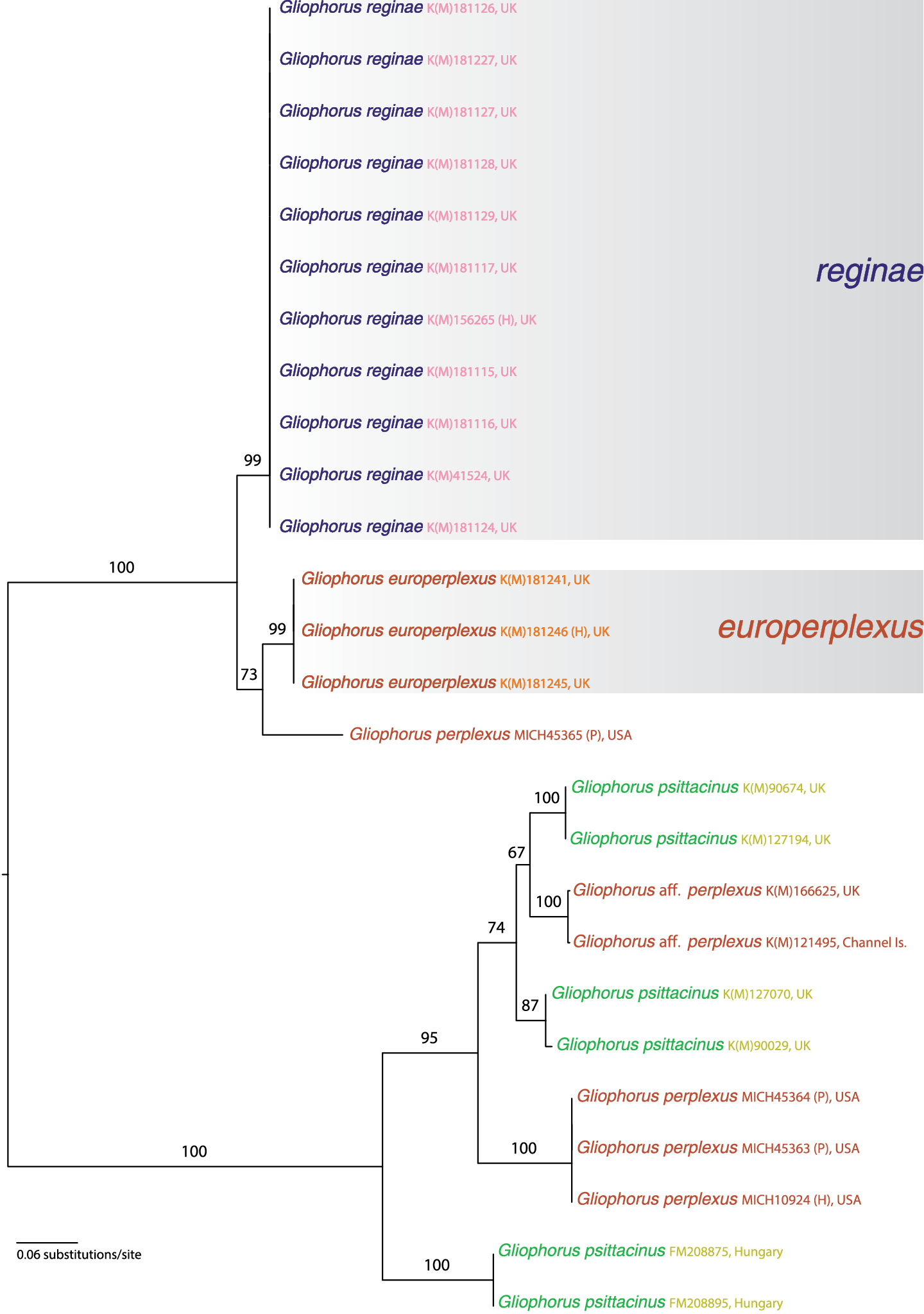
The full ITS region was amplified and sequenced for 14 specimens. Only the ITS1 region was sequenced for all specimens from MICH, while only the ITS2 region was sequenced for K(M)181124. The ITS1 and ITS2 regions were amplified and sequenced separately for K(M)181245, and the two non-overlapping regions were concatenated and separated by 66 gaps corresponding to the 5.8S ribosomal subunit in the final alignment. Phylogenetic analysis resulted in a highly resolved tree with most nodes receiving strong bootstrap support (Fig. 1). Both Gliophorus psittacinus and Gliophorus perplexus were found to be polyphyletic. Two distinct clades were strongly supported (100%): 1) Gliophorus reginae (99%) and a subclade (73%) consisting of Gliophorus europerplexus (99%) and a single sequence from a paratype specimen of Gliophorus perplexus, and 2) all other sequences, including three Gliophorus psittacinus clades (87%, 100%, 100%) comprising the GenBank sequences, one Gliophorus aff. perplexus clade (100%), and one clade composed of sequences from the holotype and two paratype specimens of Gliophorus perplexus (100%).
Maximum likelihood phylogram using full and partial nuclear ribosomal internal transcribed spacers (ITS) sequences. Numbers above branches are nonparametric bootstrap values. Tree is arbitrarily rooted at the midpoint. Two well-supported terminal clades representing the new species Gliophorus reginae and Gliophorus europerplexus are superimposed over light grey boxes. Species names for specimens from which sequences were derived are followed by fungarium or INSD accession number, and geographic location. Notations (H) and (P) indicate specimens used were holotypes or paratypes, respectively.
Registration Identifier: IndexFungorum IF550184
http://species-id.net/wiki/Gliophorus_reginae
Figures 2, 3, 6UNITED KINGDOM. England. Worcestershire (vice county 37): Bewdley, Willow Bank, 52°21.46'N, 2°22.42'W (Nat. Grid Ref. SO746733), 24 Jan 2008, R.Winnall (K(M)156265)
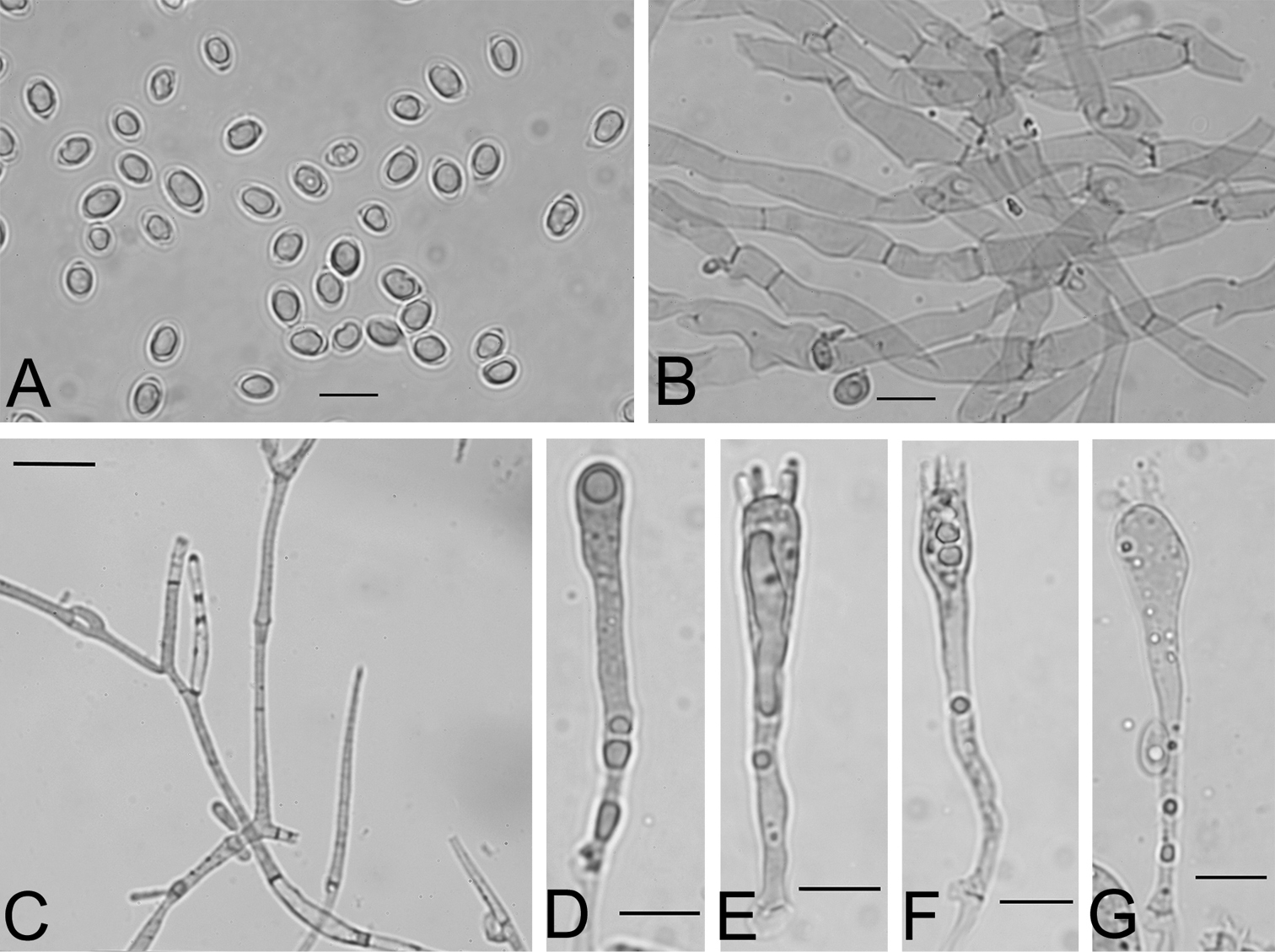
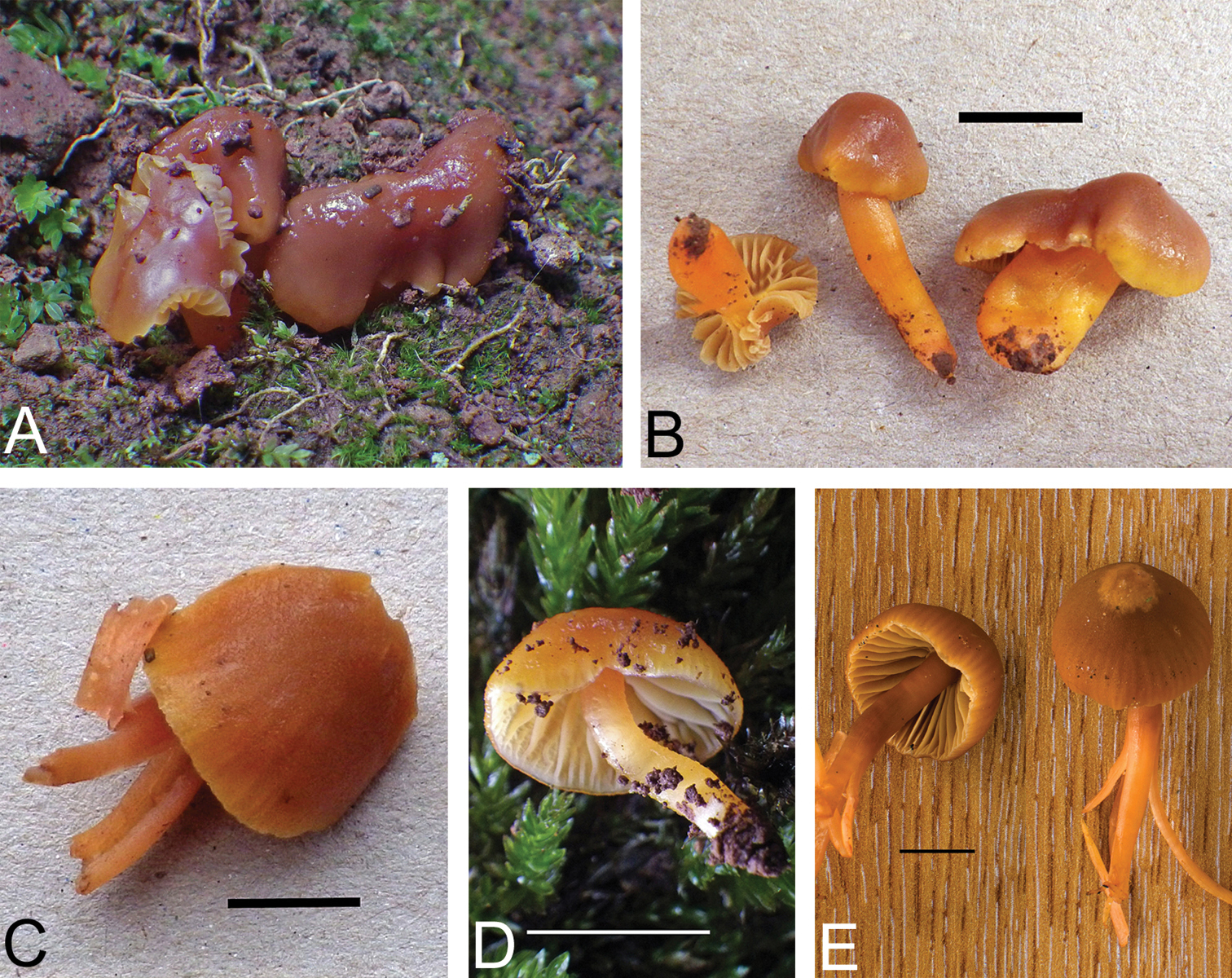
Pileus 15–55 mm diam., hemispherical to broadly conical or campanulate, initially with incurved margin, becoming applanate, often retaining broad umbo and irregular, lobed outline with indentations, folds and pleats, sometimes becoming radially furrowed, or split and flared, margin faintly to strongly translucently striate to half-way and becoming reflexed to highly revolute, viscid with gelatinous pellicle, sometimes with minutely rugose texture, at first usually dull violet purple (vinaceous grey to purple) with areas of pink, darker red or red-brown tones (rose, blood red to rusty tawny), sometimes more brownish (purplish date to dark brick), becoming paler and pinkish especially around margin which can also develop yellow (luteous) or yellow-brown (fulvous) tints, hygrophanous, dried pilei characteristically pale orange (saffron) flushed pink (rose). Lamellae ventricose, mostly narrowly adnate with some free, sinuate or broadly adnate elements, intervenose, concolorous with pileus near pileal attachment, becoming paler towards free edge, sometimes with yellow (luteous) or orange (saffron) tints. Stipe 15–70 × 5–15 mm, relatively stout, sometimes tapering upwards from the clavate base, hollow, often flexuose or tortuous, compressed or grooved, viscid but usually slightly less so than pileus, white often apically tinged with pileal colour and basally yellow (luteous) to pale orange (saffron) or becoming so, sometimes with purplish (vinaceous grey) blotches if frosted. Outer tissues of context concolorous with adjacent external surfaces, inner tissues paler. Dried lamellar trama (lens) often conspicuously dark pink (coral), contrasting with paler subhymenium and lamellar surfaces. Green pigments entirely absent. Without distinctive taste or smell. Spores [120, K(M)181126*, K(M)181127*, K(M)181128*, K(M)181129*] 6.0–8.5(-9.0) × 4.0–5.5(–6.0) µm, per-basidioma mean values 7.0–7.5 × 5.0 µm, Q=1.2–2.0, mean 1.5, short-ellipsoidal to ellipsoidal, not constricted. Basidia predominantly 4-spored, clavate, relatively long and slender with long attenuated base, (37–)40–63(–67) × 6–10 µm excluding sterigmatal length (4.0–8.0 µm). Clamp connections on basidia, within lamellar trama and pileipellis often with conspicuously looped hook cells (medallion clamps). Lamellar trama subregular with some interwoven elements, compartments 24–183 × 4–24 µm. Stipitipellis and pileipellis are ixotrichoderms.
Basidiomata of Gliophorus reginae showing pileal colour range: A–D purple, E–F pink and G–H reddish brown, scale bars represent 20 mm. Photographs C–F taken in situ at the type locality. A, B K(M)181115* photographed by R.D. Foster. C K(M)181117* and D K(M)181123 photographed by R. Winnall. E(left), F K(M)181128* and E (right) K(M)181127* photographed by A.M.A. G, H K(M)181124* photographed by D.J. Harries.
Microscopic characters of Gliophorus reginae collected from the type locality, B–G mounted in Congo Red, scale bars represent 10 µm. A Spores from water mount of spore print K(M)181127* B Subregular lamellar trama from squash mount K(M)181129* C Pileipellis hyphae showing medallion clamp connections K(M)181127* D–F Basidial developmental series K(M)181129* G Basidium K(M)181127*.
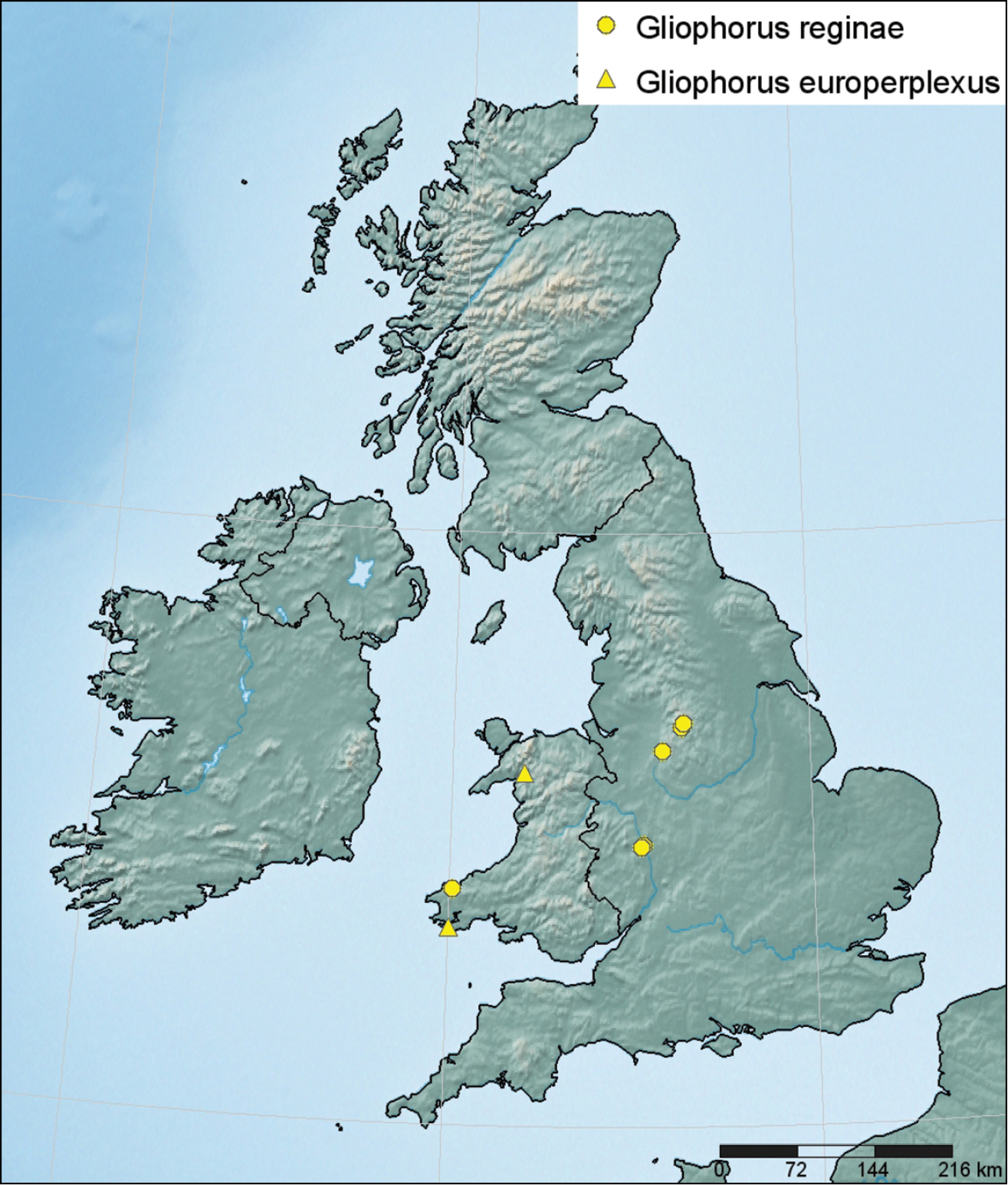
Known from a cemetery in West Wales (Pembrokeshire) and fields in central England (Worcestershire, Staffordshire and Derbyshire). The earliest known collection was made by C. Lovatt in Staffordshire in 1996 who noted that she had recorded similar specimens in 1994. It has fruited on private land at Willow Bank (Worcestershire) almost every year from 2000 onwards and recorded there in five discrete fruiting patches in a single field of ca. 0.8 ha.
In unimproved short (grazed or mown) acid-neutral rough pasture or other grassland. This species is often a relatively late fruiter and can continue producing basidiomata in January, long after other waxcaps have finished.
Latin reginae meaning “of a queen”, named for the royal purple colour of the basidiomata and to celebrate the diamond jubilee of Her Majesty Queen Elizabeth II in 2012 and the 60th anniversary of her coronation in 2013.
Collectors noted that although basidiomata of this species resembled Gliophorus psittacinus, some characters such as pileal colour and radial splitting, were more characteristic of Hygrocybe calyptriformis. Furthermore, dried collections of the latter and Gliophorus reginae often attained a similar reddish-coral tint in the fungarium that was distinct from the pale saffron of Gliophorus psittacinus. This similarity facilitated a rapid search of the British Gliophorus psittacinus collections at Kew, but no additional Gliophorus reginae specimens were discovered. This suggests that it is genuinely rare in Britain and Endangered (EN D, <250 mature individuals) might be the current regional conservation assessment following IUCN guidelines, categories and criteria (
United Kingdom. England. Derbyshire (vice county 57): Edale, Lower Hollins Farm, 53°21.84'N, 1°47.96'W (Nat. Grid Ref. SK134852), 5 Oct 2010, R.D.Foster WCS8 WX108 (K(M)181114). Ibid. 17 Oct 2010, R.D.Foster WCS15 WX115 (K(M)181115*). Woodlands Valley, Rowlee Bridge Fields, 53°23.99'N, 1°46.60'W (Nat. Grid Ref. SK149892), 9 Nov 2010, R.D.Foster WCS26 WX126 (K(M)181116*). Staffordshire (vice county 39): Danebridge (near), 53°10.25'N, 2°3.99'W (Nat. Grid Ref. SJ956637), 22 Oct 1996, C.Lovatt (K(M)41524*, sub Hygrocybe psittacina). Worcestershire (vice county 37): Bewdley, Bowcastle Farm cherry orchard, 52°22.38'N, 2°20.40'W (Nat. Grid Ref. SO769750), 3 Nov 2004, R.Winnall WX461 (K(M)181227*). Bewdley, Willow Bank, 52°21.46'N, 2°22.42'W (Nat. Grid Ref. SO746733), 4 Nov 2001, R.Winnall (K(M)92058, sub Hygrocybe cf. psittacina). Ibid. 11 Nov 2004, R.Winnall WX459 (K(M)181117*). Ibid. 15 Dec 2004, R.Winnall WX460 (K(M)181123). Ibid. 18 Oct 2012, R.Winnall WX673 (K(M)181126*). Ibid. 15 Jan 2013, R.Winnall & A.M.Ainsworth WX694 (K(M)181127*), WX695 (K(M)181128*), WX696 (K(M)181129*). Wales. Pembrokeshire (vice county 45): Fishguard Cemetery, 51°59.30'N, 4°57.64'W (Nat. Grid Ref. SM96803634), 11 Nov 2011, J.E.Hodges DJH055 WX535 (K(M)181124*).
Registration Identifier: IndexFungorum IF550185
http://species-id.net/wiki/Gliophorus_europerplexus
Figures 4–6UNITED KINGDOM. Wales. Pembrokeshire (vice county 45): Hundleton, Somerton Farm, 51°39.88'N, 4°59.54'W (Nat. Grid Ref. SM931004), 11 Oct 2012, D.J.Harries DJH064B WX664 (K(M)181246)
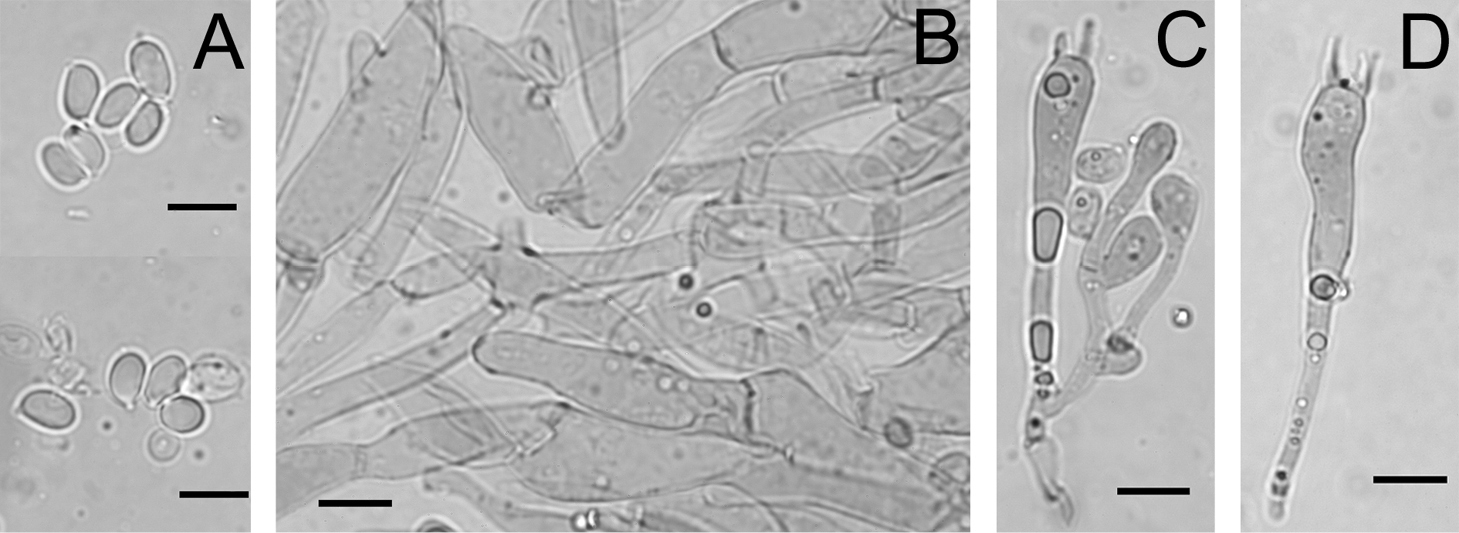
Pileus 10–25 mm diam., hemispherical to conical or campanulate, sometimes with incurved margin, becoming plano-convex or remaining broadly conical, often umbonate, sometimes with irregular, lobed outline, margin faintly to strongly translucently striate to half-way, viscid or at least very lubricous, sometimes partially flared, at first usually pink-brown to orange-brown (brick, rusty tawny to fulvous), margin paler sometimes with orange (sienna to apricot) tints, hygrophanous, dried pilei dull orange (saffron to rust). Lamellae ventricose, mostly narrowly to broadly adnate with some slightly decurrent elements, intervenose, concolorous with pileus near pileal attachment, becoming paler towards free edge. Stipe 12–60 × 2–8 mm, cylindrical or compressed, sometimes with clavate base, hollow, sometimes flexuose, viscid but usually slightly less so than pileus, apically concolorous with pileus, paler below, sometimes basally tinted orange (apricot). Dried lamellar trama (lens) often darker than subhymenium and lamellar surfaces. Green pigments entirely absent. Without distinctive taste or smell, although one specimen [K(M)181241*] was noted to have a faint rubbery smell reminiscent of Gliophorus laetus. Spores [70, K(M)181241*, K(M)181245*, K(M)181246*] (6.5–)7.0–9.0 × (4.0–)4.5–5.5(–6.0) µm, per-basidioma mean values 7.5–8.0 × 5.0 µm, Q=1.3–1.8, mean 1.6, short-ellipsoidal to ellipsoidal, not constricted. Basidia predominantly 4-spored, clavate with attenuated base, (30–)34–57 × 5–9 µm excluding sterigmatal length (3.0–7.0 µm). Clamp connections on basidia, within lamellar trama and pileipellis usually normal, occasionally with conspicuously looped hook cells (medallion clamps). Lamellar trama subregular, compartments 20–120 × 4–21 µm. Stipitipellis and pileipellis are ixotrichoderms.
Basidiomata of Gliophorus europerplexus, scale bars represent 10 mm. Photographs A–D by D.J. Harries taken in situ at, or of collectionsfrom, the type locality, and E by B.T.M.D. A, B K(M)181245* C, D K(M)181246* holotype E K(M)181241*.
Microscopic characters of Gliophorus europerplexus, A mounted in Melzer’s Reagent and B–D(holotype) in Congo Red, scale bars represent 10 µm. A Spores from lamellar squash K(M)181241*B Subregular lamellar trama from squash mount K(M)181246*C–D Basidia K(M)181246*.
Distribution of Gliophorus reginae (●) and Gliophorus europerplexus (▲) in Britain based on sequenced collections and plotted using SimpleMappr (
Identified from two sites in west Wales (Merionethshire and Pembrokeshire) supported by DNA sequence data. Fruiting was probably observed at the type locality by D.J. Harries on 6 August 2009 but no material was kept.
In unimproved short acid-neutral rough pasture in Merionethshire and found fruiting on bare soil near mosses on an almost vertical south-facing earth bank on farmland in Pembrokeshire.
Named to distinguish this European taxon from the morphologically similar Hygrophorus perplexus A.H.Smith & Hesler, a species with North American type material.
Initially, it seemed likely that historic British collections assigned to Hygrocybe psittacina var. perplexa would be redetermined as Gliophorus europerplexus following DNA sequencing. However, two specimens filed in K under the former name yielded distinct ITS sequences (Fig. 1). Therefore, the distribution of Gliophorus europerplexus is currently unknown and it should be assessed as Data Deficient.
United Kingdom. Wales. Merionethshire (vice county 48): Croesor, Cnicht, 52°58.34'N, 4°0.22'W (Nat. Grid Ref. SH64 but estimated to be SH655435 for conversion to latitude and longitude), 11 Oct 2011, E.J.M.Arnolds WX359 (K(M)181241*). Pembrokeshire (vice county 45): Hundleton, Somerton Farm, 51°39.88'N, 4°59.54'W (Nat. Grid Ref. SM931004), 19 Aug 2012, D.J.Harries DJH064A WX663 (K(M)181245*; immature).
The traditional Gliophorus (=Hygrocybe) psittacinus species concept is relatively broad (
One of the novel taxa, Gliophorus reginae, is recognisable in the field having a relatively stout stipe, sometimes yellowing at the base, and distinctive deep purple or reddish-brown pileus. Our field observations suggest that a colour form of this might be shown in Boertmann’s photograph of Danish specimen DB 2000/33 taken at Lysnet, E. Jylland, on 17 Oct. 2000 (
In Europe, Hygrophorus sciophanus (Fr.) Fr. is currently regarded as a synonym of Hygrocybe psittacina var. perplexa with Hygrophorus perplexus A.H. Sm. & Hesler as basionym. By contrast,
Our analysis showed that the ITS sequence derived from the holotype specimen of Hygrophorus perplexus is certainly distinct from the second of our new species, Gliophorus europerplexus. Two specimens identified as Hygrocybe psittacina var. perplexa (Table 1) collected in 2003 and 2008 were also sequenced, but they are phylogenetically distinct, forming a clade near to Gliophorus psittacinus (Fig. 1) and may represent a further novel taxon. The single anomalous sequence from a paratype of Hygrophorus perplexus, which comes near Gliophorus europerplexus in our analysis, reveals additional cryptic diversity within this species complex in North America and highlights the difficulty in correctly naming waxcap species using morphology alone. Attempts should be made, therefore, to sequence additional European and North American specimens currently filed as Gliophorus perplexus, Hygrophorus perplexus, Hygrocybe perplexa and Hygrocybe psittacina var. perplexa to gain a better understanding of the distribution of Gliophorus europerplexus and other emerging segregate taxa.
We would like to thank Defra, Natural England and Scottish Natural Heritage for financial support and all those who collected and sent specimens of the two species described herein: E.J.M. Arnolds, R.D. Foster, D.J. Harries, J.E. Hodges and R. Winnall. Thanks also to R.D.F., D.J.H. and R.W. for allowing us to use their photographs.