(C) 2013 Cristina Máguas. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Due to the close linking between the biosphere and atmosphere, there are clear impacts of changes in climate, atmospheric deposition of nutrients/pollutants and land use (Global Changes) on the terrestrial biosphere. Lichens, with a direct dependence on atmospheric conditions, are much more affected by their immediate microclimate than by the ecosystem’s prevailing macroclimate. In contrast to higher plants, poikilohydric organisms have different mechanisms of water and CO2 exchange. The application of stable isotopes to the understanding of the mechanisms that are fundamental to lichen gas exchange and water uptake is a promising tool for the evaluation of lichen response to environmental changes. Indeed, lichens have been shown to be influenced by a large number of natural and anthropogenic environmental factors, serving as ecological indicators. Thus, we may use these organisms to model the impact of key global change drivers, such as nitrogen deposition and biodiversity changes, at local scale. Particularly useful is the application of the Lichen Diversity Value (LDV) in order to evaluate the impact of global drivers. Moreover, it has been shown that these indices, associated with main photobiont types, green-algae (LDVch) or cyanobacteria (LDVcyh), and/or nitrophilous versus oligotrophic species, were good candidates as ecological indicators. Besides mapping with high spatial resolution the effects of climate alterations, lichen functional groups could also be used as an early-warning system in order to detect the first effects of climate change in ecosystems before sudden shifts occur on other components that may be less sensitive. Clearly, lichens possess the adequate traits to be used as powerful indicators of complex interactions between atmosphere and biosphere, and thus can generate potentially interesting models for global change drivers.
Climate change, Ecology, Photobionts, Physiology
The biosphere has a significant impact on the composition of the atmosphere, and the interaction between biosphere and atmosphere affects all living organisms, including humans. Changes in climate, atmospheric deposition of nutrients/pollutants and land use (Global Changes) have observable impacts on the terrestrial biosphere (i.e.
Given the close linking between the biosphere and atmosphere, the exchange of carbon (CO2) and water vapor between biosphere and atmosphere, and the deposition of nutrients and heavy metals to the plant or ground surface are very important areas of research. Research in this area involves measurements of the exchange of gases (i.e. CO2 and water vapor) using eddy covariance (and other) flux techniques, spectroscopic and tracer release methods (for trace gases such as N2O) , as well as satellite data and modeling approaches (i.e.
Approximately 8% of the earth’s land surface is covered by vegetation types dominated by lichens (
All environments with a scarcity of nutrients or water supply are dominated by poikilohydric cryptogams such as bryophytes (mosses and liverworts) and lichens. Over 33% of the Earth’s surface is covered by semiarid and arid lands dominated by biological soil crusts composed mainly of cyanobacteria and lichens (
The use of stable isotopes is a powerful research tool in environmental sciences. Its combination with: i) concentration measurements (providing “Keeling-type” plots;
Due to their poikilohydric nature and the lack of stomatal control, lichens do not show the typical stable isotope compositions (d13C) of higher plants. The d13C value of their organic matter (OM) vary between -12 and -23 ‰, and such a high d13C heterogeneity is thought to be due to discriminating factors such as the CO2 diffusion resistance and CO2 source, further than photosynthetic RuBisCO (Ribulose-1, 5-bisphosphate carboxylase oxygenase) fractionation (
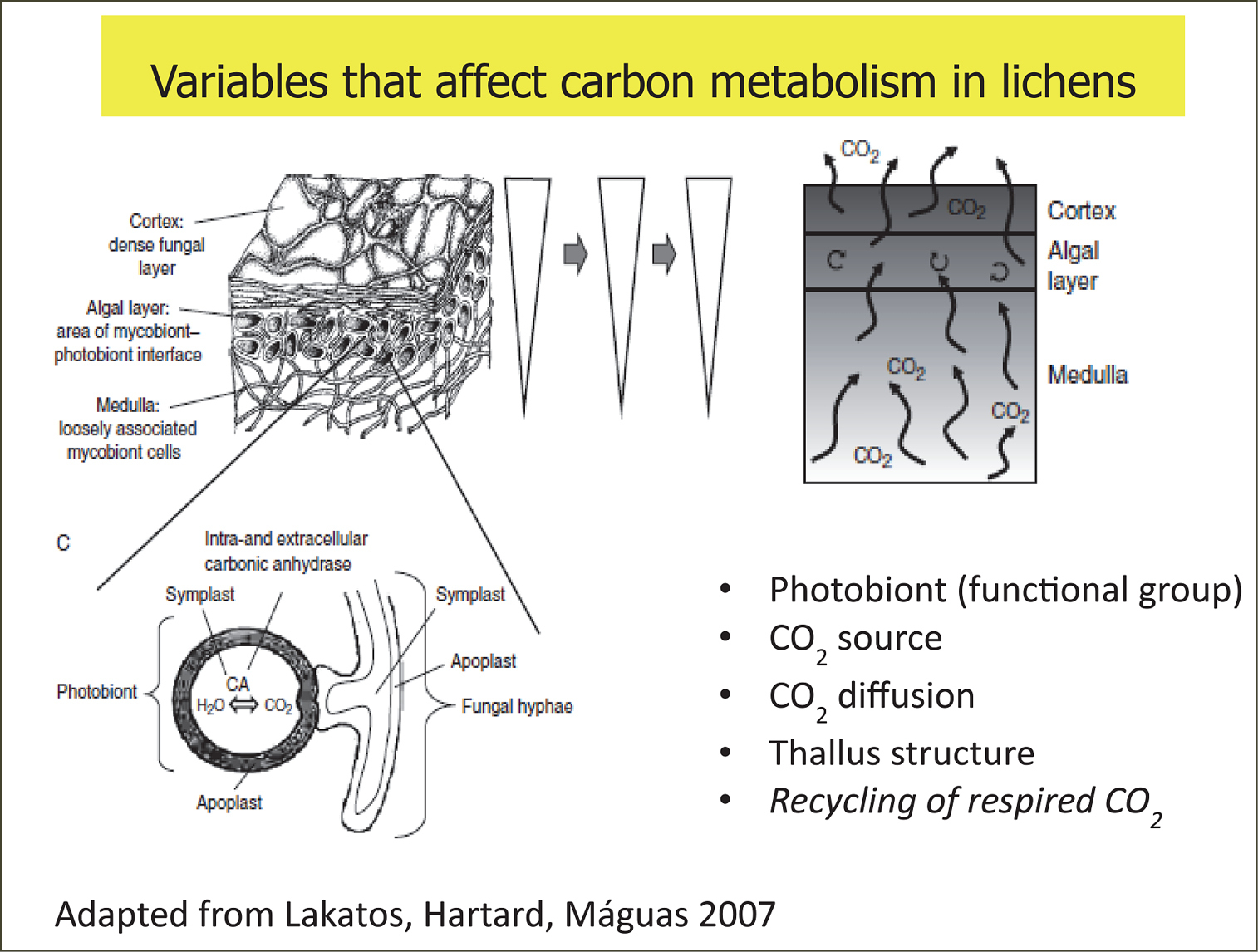
Schematic illustration of a cross-section through a lichen thallus with distinctive cortex, algal layer and medulla and the main factors contributing to thallus CO2 exchange and the consequence for carbon d13C fractionation that affects organic matter in lichens.
Over the surface of a single thallus CO2 diffusion resistances can be modified by morphological structures such as the conglutinated cortical layers, thallus thickness and density and concomitant structural changes during water absorption. Hence, thinner thallus regions such as margins or tips, which are often loosely constructed and displaying lower CO2 diffusion resistances, should also lead to lower d13C discrimination (depleted d13C). However, the observed high heterogeneity of d13C of margins versus center parts within different growth forms and photobiont groups (from 0.25 to 2.5 ‰) does not give a clear indication of the expected discrimination factor associated with morphology and thallus structure, and no correlation with respect to growth form has been assessed (
Besides the major photosynthetic fractionations due to the transport and fixation of CO2, one crucial factor influencing δ13C is the origin of the carbon source used by lichens in a specific microenvironment. Several factors may contribute to this: i) depending on where they are located lichens can be attached to a substratum where the most direct CO2 source is not atmospheric CO2; and ii) different lichen species or different individual thalli might fix respired CO2 from different ecosystem components . For example, it is well established that in macrohabitats such as closed forests, the source of ambient CO2 gradually changes with height from the forest floor to the canopy (the vertical CO2 profile) (e.g.
As mentioned earlier, poikilohydric cryptogams can have a major influence on water and CO2 fluxes in an ecosystem but their mechanisms of water exchange differ to those of higher plants. Their water status, for example, varies passively with surrounding environmental conditions, and they have neither a continuous influx of water nor stomata to control water deficit. Hence, during evaporation no isotopic steady state can be achieved and the d18O composition of both the thallus water and the evaporated water is expected to show progressive enrichment similar to the Rayleigh distillation process. As the water also transduces its oxygen isotopic signal to CO2via hydration of dissolved CO2 (e.g.
As a model organism, the globally distributed lichen Cladina arbuscula was studied under laboratory conditions as well as in the field. During a desiccation experiment, δ18O values of thallus water and respired CO2 became enriched by ~ 7‰ and followed an enrichment pattern similar to that of higher plants. However, the observed degree of enrichment was lower in comparison to higher plants due to (i) the lichen’s inherent lower evaporative resistances and (ii) a stronger effect of the more depleted surrounding water vapor (
The use of lichens as ecological-indicators of biogenic fluxes between biosphere and the atmosphere
Lichens, which rely largely on the atmosphere for water and nutrient supply, can be used as suitable indicators of environmental changes in terrestrial ecosystems (
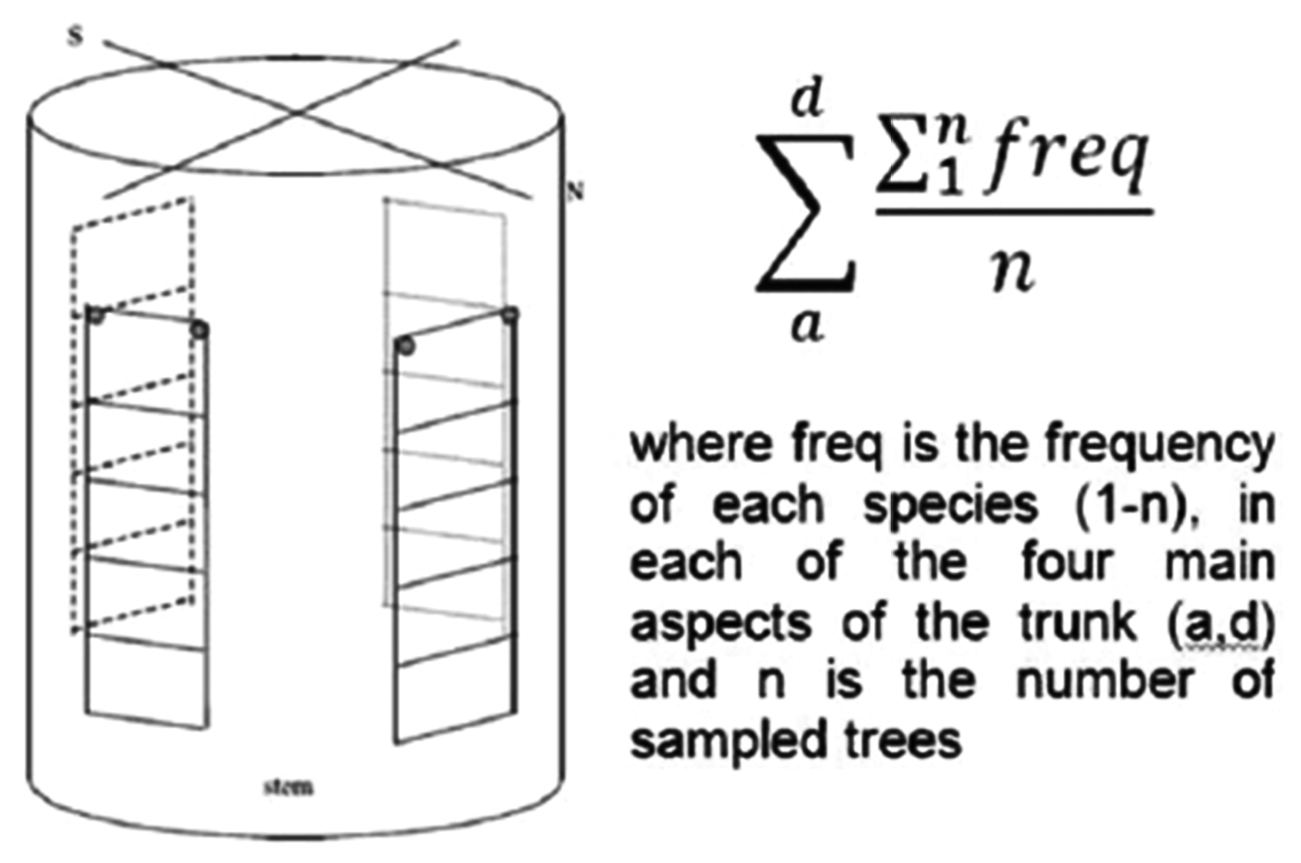
Distribution of the sampling grid in a tree trunk, original picture (
An alternative to total diversity is to use of functional traits, such as lichen photobiont type, eutrophication and water stress tolerance, which is strongly involved in species responses to several environmental factors. Indeed, in a study conducted in a Mediterranean Quercus faginea subsp broteroi forest, the lichen functional diversity was highly significantly related to potential solar radiation, an integrated measure of long-term microclimate. The green-algal lichens were positively related to potential solar radiation whereas cyanolichens were negatively related (
Lichens can also be classified according to their preferences or tolerance to eutrophication (
Since lichens are directly influenced by microclimatic conditions, such as light, water, temperature and CO2 concentration, the isotopic composition of their OM integrates environmental factors acting on their specific microhabitat over a range of weather conditions, as well as a variety of land-uses over long periods of time. The isotopic composition of OM is determined by an economic equilibration between carbon source and sink, which are mainly photosynthesis and respiration. Although the mycobiont dominates the OM pool, the carbon acquisition of the lichen depends on the water content, light intensity and CO2 fixation of the photobiont. Carbon isotope discrimination processes of 13C can thus be related to CO2 acquisition modes, CO2 diffusion and CO2 sources. To summarize, in several microhabitats, respired d13C-depleted CO2 serves as the carbon source for photosynthesizing lichens, thus biasing their characteristic isotopic signatures, which otherwise are determined by physiological processes. Thus, lichens can be used as tracers to point out the prevailing CO2-sources in microhabitats. Therefore lichen OM will also indicate, especially in the younger thallus parts, an alteration in ambient 13C-CO2 caused by changes of urban-rural and land-use boundaries.
Moreover, the well-identified poikilohydric natures of these organisms make them sensitive tracers of water vapor fluxes. Indeed, application of the stable oxygen isotope ratio (δ18O) to gain insights into the yet unknown fractionation processes of terrestrial poikilohydric organisms showed that thallus water isotopic composition is additionally influenced by the prevailing environmental conditions to which they are exposed to. Thus, we may use these results to assess the effect of a substantial lichen ground cover on water exchange processes between the soil and atmosphere. Numerous nutrient poor habitats in extreme climates are dominated by poikilohydric organisms. Hence, globally, the effects of these organisms on overall water fluxes may even be more remarkable.
Lichen communities can thus be used to study important interactions between atmosphere and biosphere. Accordingly, the concomitant application of geostatistical models and lichen functional diversity (green algal and cyanobacterial LDV indexes) are a suitable way to use lichen communities as good indicators of complex interactions between atmospheric nutrients deposition (i.e. atmospheric NH3) and the biosphere, as well as microclimatic changes due to forestry and land-use practices.
The research associated with the carbon and water stale isotope studies was supported within the EC-program NETCARB (HPRN-CT-1999-59), the ESF-program SIBAE (62561 EXGC EX03), the German and Portuguese Academic Exchange Service DAAD/GRICES (PPP- DAAD/GRICES; D/04/42019) and the Portuguese Science Foundation (POCTI/BIA-BDE/60140/2004). The evaluation of the lichens as ecological tracers were financed by: the Portuguese Foundation for Science and Technology (FCT-MCTES) within the project –Effects of fragmentation on stand structure of Quercus faginea forests: key factors influencing water balance (POCTI/BIA-BDE/60792/2004); European Union, within the project –BIOASSESS, Development of Biodiversity Assessment Tools (EVK4-1999-00280); European Science Foundation NinE program, COST Action 729 (Assessing and Managing nitrogen fluxes in the atmosphere-biosphere system in Europe), within the EC NitroEurope Integrated Project. The authors would like to thank Rodrigo Maia for technical assistance with the stable isotopic analysis.