(C) 2012 Paul Kirika. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Abstract
The new sorediate species Lecanora kenyana from Mount Kenya and Lecanora orientoafricana from the Rift Valley in Kenya are described. Lecanora kenyana has red-brown apothecia with a constricted base, a melacarpella–type amphithecium, pulicaris–type epihymenium, a hyaline hypothecium, and contains usnic acid as major constituent. Lecanora orientoafricana is characterized by having a dark hypothecium, pulicaris-type amphithecium, chlarotera-epihymenium, and contains atranorin and gangaleoidin. A phylogenetic analysis using maximum likelihood and a Bayesian approach based on DNA sequence data of mtSSU and ITS rDNA support that both new species belong to Lecanora sensu stricto and cluster with species containing usnic acid or having a dark hypothecium, respectively.
Kenya, Lecanorales, new species, taxonomy, tropical lichens
Lecanora is the major genus of Lecanoraceae (
The study is based on material deposited in EA and F and DNA sequences downloaded from Genbank. Sequences of five Ramboldia spp. were included as outgroup since the genus has been shown previously to be related to Lecanora (
Species and specimens used in the present study. Newly obtained sequenced in bold.
| Species | Collection data | mtSSU acc. no. | ITS acc. no |
|---|---|---|---|
| Lecanora achroa | JQ782663 | JN943714 | |
| Lecanora aff. achroa | JQ782662 | JQ782708 | |
| Lecanora allophana | AY567710 | AF070031 | |
| Lecanora argentata | JQ782664 | JQ782704 | |
| Lecanora argopholis | DQ787358 | JQ782705 | |
| Lecanora austrotropica | JQ782665 | JQ782706 | |
| Lecanora californica | JQ782668 | JQ782707 | |
| Lecanora campestris | DQ787362 | AF159930 | |
| Lecanora elatinoides | JQ782669 | JQ782709 | |
| Lecanora flavopallida1 | JQ782673 | JN943723 | |
| Lecanora flavopallida2 | JQ782674 | JN943724 | |
| Lecanora flavoviridis | JQ782675 | JQ782711 | |
| Lecanora gangaleoides | JQ782676 | JQ782712 | |
| Lecanora helva1 | JQ782679 | JQ782716 | |
| Lecanora helva2 | JQ782680 | JQ782715 | |
| Lecanora aff. helva | JQ782677 | JQ782713 | |
| Lecanora aff. helva | JQ782678 | JQ782714 | |
| Lecanora hybocarpa | DQ912273 | DQ782849 | |
| Lecanora kenyana | Kenya, Kirika 1179 (F) | JQ900616 | JQ900618 |
| Lecanora leproplaca1 | JQ782683 | JQ782718 | |
| Lecanora leproplaca2 | JQ782684 | JQ782719 | |
| Lecanora leprosa1 | JQ782682 | JQ782721 | |
| Lecanora leprosa2 | JQ782685 | JQ782720 | |
| Lecanora orientoafricana | Kenya, Kirika 2205 (F) | JQ900617 | JQ900619 |
| Lecanora pacifica | JQ782686 | JQ782722 | |
| Lecanora paramerae | EF105418 | EF105413 | |
| Lecanora phaeocardia1 | JQ782687 | JQ782724 | |
| Lecanora phaeocardia2 | JQ782688 | JQ782723 | |
| Lecanora plumosa1 | JQ782689 | JQ782725 | |
| Lecanora plumosa2 | JQ782690 | JQ782726 | |
| Lecanora pseudogangaleoides ssp. verdonii | JQ782691 | JQ782727 | |
| Lecanora queenslandica | JQ782692 | JQ782728 | |
| Lecanora sp.C | JQ782693 | JQ782729 | |
| Lecanora sp.E (sorediate) | JQ782694 | JQ782730 | |
| Lecanora subimmergens1 | JQ782696 | JQ782732 | |
| Lecanora subimmergens2 | JQ782695 | JQ782731 | |
| Lecanora subimmersa | JQ782697 | JQ782733 | |
| Lecanora toroyensis | JQ782698 | JQ782734 | |
| Lecanora tropica | JQ782699 | JN943720 | |
| Lecanora ulrikii | JQ782700 | - | |
| Lecanora vainioi1 | JQ782702 | JN943716 | |
| Lecanora vainioi2 | JQ782701 | JN943717 | |
| Lecanora wilsonii | JQ782703 | - | |
| Ramboldia arandensis | EU075527 | EU075541 | |
| Ramboldia brunneocarpa | EU075528 | EU075542 | |
| Ramboldia laeta | EU075530 | EU075544 | |
| Ramboldia petraeoides | EU075531 | EU075545 | |
| Ramboldia stuartii | EU075535 | EU075549 |
Alignments were done using Clustal W (
Anatomical studies were conducted using standard light microscopy on hand-cut sections mounted in water. Secondary lichen substances were identified by high performance thin-layer chromatography (HPTLC) according to the standard methods (
The data underpinning the analyses reported in this paper are deposited in the Dryad Data Repository at doi: 10.10.5061/dryad.b1068.
Results and discussion The SpeciesKenya, Eastern Prov., Mount Kenya National Park, Chogoria Track, close to Chogoria Gate, open Juniperus-Podocarpus woodland, 0°09'S, 37°26'E, 2960m alt., 27.01.2010, on Juniperus, P. Kirika 1179, G. Mugambi & H.T. Lumbsch (holotype EA, isotype F).
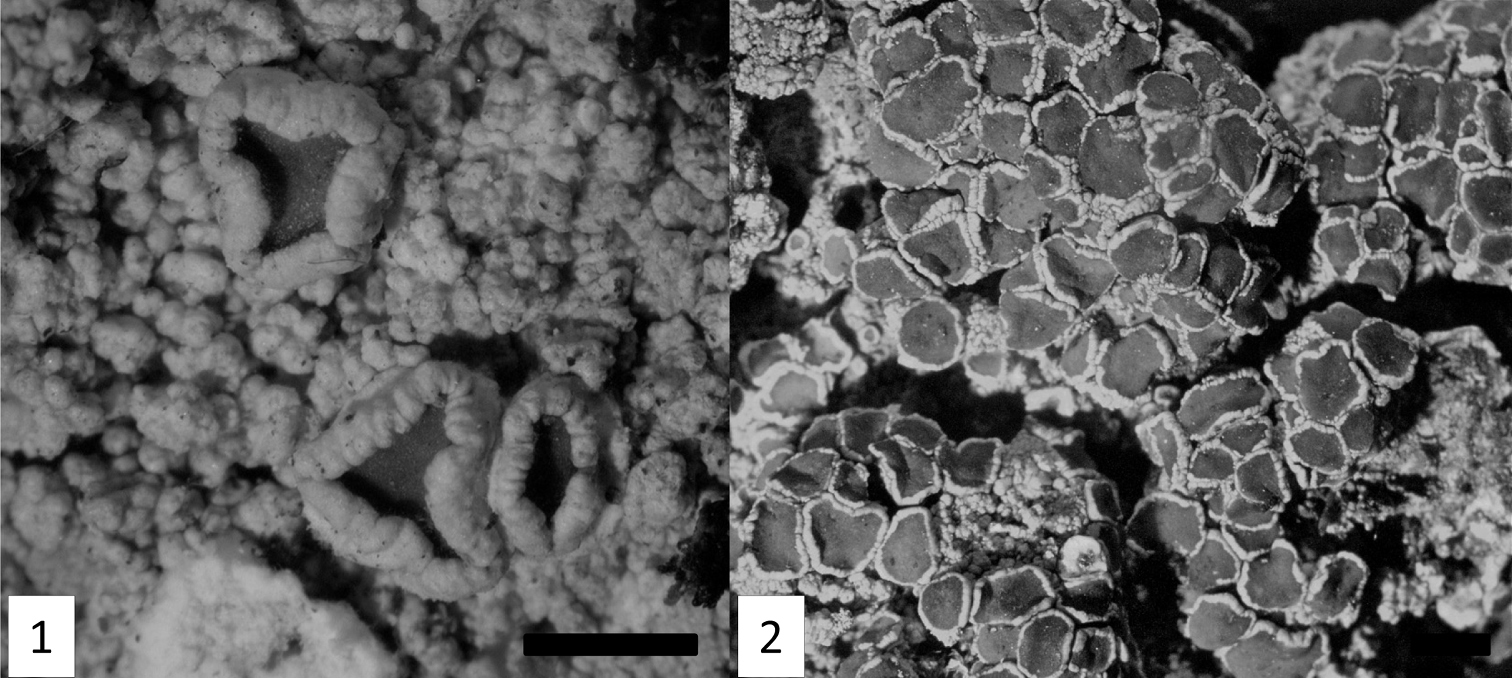
Thallus crustose, verrucose to verruculose, thin to thick, glossy, whitish to greenish; margin indistinct; prothallus not visible; sorediate. Soralia roundish, concave, 0.5–1.2 mm diam., remaining distinct or coalescing, with granular soredia, yellowish green to yellowish gray. Apothecia sessile, strictly constricted at base, 0.6–2.0 mm diam., lecanorine; disc red-brown, shiny, plane, epruinose; margin concolourous with thallus, prominent, thick, smooth, entire to verruculose, flexuose. Amphithecial cortex uniform, gelatinous, inspersed with crystals, hyaline, 25–45 µm thick, with hyphae growing out basally. Amphithecium with small and large crystals (=melacarpella-type). Hypothecium hyaline, 25–30 µm high, parathecium hyaline, with yellowish crystals, 5–7 µm thick. Hymenium hyaline, 55–70 µm high, clear. Epihymenium red–brown, 10–12 µm thick, with numerous, small crystals; pigmentation and crystals dissolving in K (=pulicaris–type). Paraphyses sparingly branched, apically slightly swollen, hyaline. Asci clavate, 50–60 × 10–14 µm, 8–spored. Ascospores ellipsoid to narrowly ellipsoid, 12–17 × 4.5–6.5 µm. Pycnidia not seen.
Morphology of the new Lecanora species. 1 Lecanora kenyana, isotype (F). 2 Lecanora orientoafricana, isotype (F). Scale bars = 1mm
Thallus and apothecial margin K+ yellow, C-, KC–, containing atranorin (minor), and usnic acid (major).
The new species is named after the country Kenya where the new species has been found.
Lecanora kenyana is characterized by relatively large, red-brown apothecia with a constricted base, a melacarpella-type amphithecium, pulicaris-type epihymenium, the presence of usnic acid as major constituent, and the presence of soralia. There are only few sorediate Lecanora sensu stricto species with usnic acid, including Lecanora brodoana, Lecanora elatinoides, Lecanora floridula, Lecanora jamesii, Lecanora mobergiana, and Lecanora transvaalensis (
At present this species is known from bark of juniper trees in open habitats at altitudes above 2800m in forests dominated by Hagenia and Podocarpus. Associated lichens included Heterodermia leucomelos, Lecanora caesiorubella, Leptogium laceroides, Lobaria pulmonaria, Pannaria fulvescens, Physcia albata, Pseudocyphellaria aurata, Physcia crocata, Varicellaria velata, and several Usnea spp.
Kenya: Eastern Prov., Mt. Kenya National Park, Sirimon route, ca. 3km for KWS gate towards Old Moses Camp, 00°00'N, 37°15'E, mature montane forest with Podocarpus, Olea, Hagenia and Arundinaria alpina, 2870m, on bark, 7.10.2010, P. Kirika 2051, G. Mugambi, G. Gatere and M. Mutembei (EA).
http://species-id.net/wiki/Lecanora_orientoafricana
Figure 2Kenya, Rift Valley Prov., Cherangani Hills, Kerer forest, degraded montane forest, 3240m, on bark, 25.07.2011, P. Kirika 2205 (EA, holotype, F-isotype).
Thallus crustose, verrucose to verruculose, thin to thick, glossy, whitish to greenish grey; margin indistinct; prothallus not visible; sorediate. Soralia roundish, 0.3–1.0 mm diam., with granulose soredia, light pale greenish white to grayish green. Apothecia sessile, constricted at base, 0.4–1.4 mm diam., lecanorine; disc light red-brown to brown, matt, plane or concave, sparsely grayish pruinose; margin concolourous with thallus, prominent, thick, smooth, verruculose. Amphithecial cortex uniform, gelatinous, inspersed with crystals, hyaline, 20–30 µm thick. Amphithecium with large crystals (=pulicaris–type). Hypothecium red-brown to yellowish brown, 30–40 µm high, parathecium hyaline, lacking crystals, 5–7 µm thick. Hymenium hyaline, 70–85 µm high, clear. Epihymenium red–brown, 10–12 µm thick, with coarse crystals; pigmentation and crystals dissolving in K (=chlarotera–type). Paraphyses sparingly branched, apically slightly swollen, hyaline. Asci clavate, 50–60 × 10–12 µm, 8–spored. Ascospores ellipsoid to broadly ellipsoid, 12.5–15.5 × 6.0–8.5 µm. Pycnidia not seen.
Thallus and apothecial margin K+ yellow, C-, KC–, containing atranorin and gangaleoidin.
The new species is named after the area East Africa where it has been collected.
Lecanora orientoafricana is characterized by the presence of granular soredia, sparsely pruinose, brown apothecia, a pulicaris-type amphithecium, chlarotera-type epihymenium, dark hypothecium, broadly ellipsoid ascospores, and the presence of atranorin and gangaleoidin. Soredia are rare among Lecanora sensu stricto species with a dark hypothecium. Some specimens of Lecanora coronulans are sorediate, but this species is readily distinguished by epruinose apothecial discs, an egranulose epihymenium, and the presence of protoconstipatic acid and zeorin and major constituents in addition to atranorin (
This new species is currently only known from the type locality in the Rift Valley province of Kenya, where it was found growing on bark in a degraded montane forest dominated by Podocarpus falcatus, Rapanea melanophloes and Faurea saligna at an altitude of 3240m. Associated species included Sphaerophorus melanocarpus, Pannaria cf. rubiginosa, and Ramalina spp.
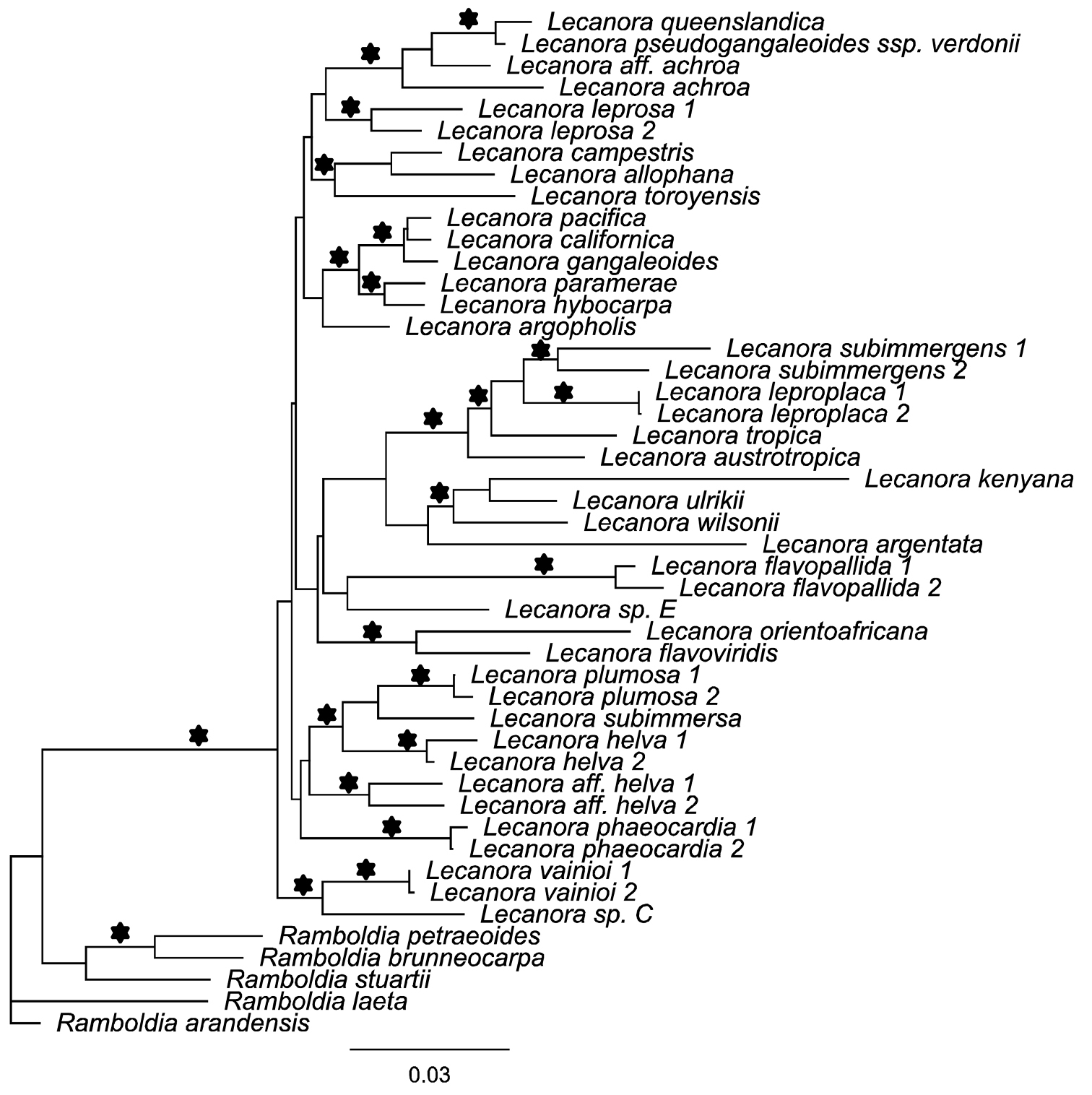
Four new sequences were generated for this study and aligned with sequences downloaded from Genbank (Table 1). The single gene locus did not show any conflicts and hence the concatenated data set was analyzed. Our combined data set included 820 unambiguously aligned positions, 174 of which were constant. The ML tree had a likelihood value of –3718.083 and in the B/MCMC analysis of the combined data set, the likelihood parameters in the sample had the following mean (Variance): LnL = -3794.172 (0.21). The ML tree and the tree from the B/MCMC tree sampling were almost identical with no differences in well-supported clades. Thus, only the ML tree is shown here (Fig. 3). In our analysis, species of the genus Lecanora form a strongly supported monophyletic group as in a previously published study (
Phylogenetic placement of the two new Lecanora species as inferred from a concatenated alignment of mtSSU and ITS DNA sequences. This is the optimal tree under maximum likelihood. Branches in bold received likelihood bootstrap support values above 70%, and posterior probabilities equal or above 0.95
We wish to thank the Kenya Wildlife Service (KWS) and the Kenya Forest Service (KFS) for providing collecting permits and Nature Kenya for partially supporting fieldwork in the Rift valley. We also thank George Mugambi (Nairobi) for his help and support on field trips. This study was financially supported by The Field Museum/IDP Foundation, Inc. African Training Fund. The laboratory work was done at the Pritzker Laboratory for Molecular Systematics and Evolution at the Field Museum.