Research Article |
|
Corresponding author: Martin Westberg ( martin.westberg@nrm.se ) Academic editor: Gerhard Rambold
© 2015 Martin Westberg, Einar Timdal, Johan Asplund, Mika Bendiksby, Reidar Haugan, Fredrik Jonsson, Per Larsson, Göran Odelvik, Mats Wedin, Ana M. Millanes.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Westberg M, Timdal E, Asplund J, Bendiksby M, Haugan R, Jonsson F, Larsson P, Odelvik G, Wedin M, Millanes AM (2015) New records of lichenized and lichenicolous fungi in Scandinavia. MycoKeys 11: 33-61. https://doi.org/10.3897/mycokeys.11.6670
|
Abstract
Fourteen species of lichenized or lichenicolous fungi are reported new to either Norway or Sweden or both countries. Several of these are rare and almost unknown. The reported species are: Acarospora insignis (new to Norway), A. pyrenopsoides (Norway, Sweden), A. versicolor (Norway), Calvitimela perlata (Sweden), Lecidea degeliana (Sweden), Nephroma helveticum (Sweden), Peltula placodizans (Norway), Phacographa protoparmeliae (Norway), Rhizocarpon pycnocarpoides (Norway, Sweden), Sarcogyne algoviae (Sweden), Sarcogyne hypophaeoides (Norway, Sweden), Tephromela grumosa (Norway), Tremella lobariacearum (Norway) and Tremella wirthii (Sweden). In addition Cladonia albonigra is confirmed from Sweden. Sarcogyne hypophaeoides is lectotypified and is also reported new to Austria.
Key words
Acarosporaceae , barcode, floristics, ITS, lichens
Introduction
Although studies of the biodiversity in Scandinavia have a long and continuous tradition, several thousands of species remain to be discovered, many of which are likely to be common. Among many of those discovered, almost nothing is known about where they occur, how they live and what their functional roles in the ecosystems are. To explore this poorly known biodiversity, the Swedish Taxonomy Initiative (STI) was established in 2002, with the aim to have all multicellular plants, fungi and animals in Sweden identified and described. Numerous taxonomic projects have been funded by the STI to investigate poorly known groups of organisms. Since 2002, almost 2000 species have been found new to Sweden and almost 1000 are new to science (
Material and methods
Material. We studied material collected during field surveys (2011–2014) funded through STI and NTI projects as well as specimens of the same species on loan from various herbaria (
Morphology. Macromorphological traits were observed using a Zeiss Stemi 2000-C dissecting microscope. Microscopical examinations were carried out using squash preparations, hand-cut sections and freezing microtome sections cut at 16 µm. The preparations were observed in distilled water, lactophenol cotton blue, or 10% KOH (K). Microscopic structures in heterobasidiomycetes were studied using handmade sections stained with Phloxin (1% in water) after pre-treatment with KOH (5%), following the methods of
Secondary chemistry. Selected specimens were examined by thin-layer chromatography (TLC), performed in accordance with the methods of
DNA barcoding. Some specimens (Table
List of sequenced specimens with Accession numbers in the European Nucleotide Archive or GenBank.
| Species | Origin | Voucher | Acc. No. (ITS) |
|---|---|---|---|
| Acarospora insignis | Norway, Oppland | Haugan 10022 ( |
LN890273 |
| Norway, Oppland | Westberg ( |
LN890274 | |
| Acarospora pyrenopsoides | Norway, Aust-Agder | Timdal 11308 ( |
LN890275 |
| Acarospora versicolor | Norway, Buskerud | Westberg 08-092 ( |
LN890276 |
| Norway, Oppland | Westberg 08-205 ( |
LN890277 | |
| Nephroma helveticum | Sweden, Västerbotten | Jonsson 4200 ( |
LN890278 |
| Norway, Oppland | Klepsland JK11-L559 ( |
KT800006 | |
| Norway, Buskerud | Hofton 13176 ( |
KT800007 | |
| Rhizocarpon pycnocarpoides | Norway, Sør-Tröndelag | Bendiksby et al. 12630 ( |
KR780560 |
| Norway, Oppland | Rui & Timdal 12665 ( |
KT800002 | |
| Norway, Buskerud | Rui & Timdal 12854 ( |
KT800003 | |
| Norway, Nordland | Haugan 11125 ( |
KT800004 | |
| Norway, Nord-Trøndelag | Haugan 11128 ( |
KT800005 | |
| Tephromela grumosa | Sweden, Bohuslän | Haugan 11501 ( |
KR303667 |
The species
Acarospora insignis
Acarospora insignis H.Magn. Svensk Bot. Tidskr. 18: 329. 1924.
Holotype
SWEDEN. Härjedalen: Viken. 1878, P. J. Hellbom (
Distribution
New to Norway. This species has until now only been known from the type collection from central Sweden.
The Norwegian specimens were both collected on siliceous boulders in open spruce forests. The type specimen grows on Aspicilia cinerea and
Specimens examined
NORWAY. Oppland: Lillehammer, Døsgrenda, between Kinnlia and Åsen, alt. 500 m, 61°05.21'N, 10°20.32'E. 1 June 2011, Haugan 10022 (
Acarospora pyrenopsoides
Acarospora pyrenopsoides H.Magn. Acta Horti Gothob. 2: 74. 1926.
Holotype
GREENLAND. Nennese. [undated], J. Vahl (
Distribution
New to Norway and Sweden. This is another poorly known species that has received very little attention since
Acarospora pyrenopsoides appear to prefer humid localities. The locality in Norway reported here, lies near a waterfall where the species was found on sloping rocks. It is the only European site of the North American Rhizocarpon bolanderi (Tuck.) Herre, and was found during an inventory of that species. The two Swedish localities are located on or near lakeshores in the province of Värmland in central Sweden.
Specimens examined
NORWAY. Aust-Agder: Valle, Hallandsfossen. 28 July 2010, Timdal 11308 (
Acarospora versicolor
Acarospora versicolor Bagl. & Carestia. Comm. Soc. Critt. Ital. 1: 440. 1863.
Type
ITALY. Piemonte. F. Baglietto s.n. (the location of the type is unknown according to
Distribution
New to Norway. Acarospora versicolor is widespread on both siliceous and calcareous rocks in Europe and western Asia and is in the Nordic countries known from one locality in Finland and one unconfirmed report from Denmark (
Acarospora versicolor belongs to the morphological group of brown Acarospora species lacking secondary metabolites. There are many names and many taxonomical problems in this group but A. versicolor was recently discussed and described in detail by
The Norwegian specimens reported here all grow calcareous rocks in sun-exposed habitats in the southern parts of the country. It is as far as we know the only one in this group of species in Scandinavia growing on calcareous rocks. However, elsewhere in Europe it also grows on non-calcareous rocks (
Specimens examined
NORWAY. Buskerud: Hole, west side of the island Storøya, 60,04685°N, 10,2376°E. 8 June 2008, Westberg 08-092 (
Calvitimela perlata
Calvitimela perlata (Haugan & Timdal) R. Sant. Lichen-forming and lichenicolous fungi of Fennoscandia: 73. 2004.
Basionym
Tephromela perlata Haugan & Timdal, Graphis Scripta 6(1): 22 (1994).
Holotype
NORWAY. Sør-Trøndelag: Oppdal municipality, Drivdalen, by the rapids in the lower part of the river Kaldvella, 62°17'N, 9°35'E, alt. 940–980 m, exposed rock face in the subalpine region. 23 July 1993, E. Timdal 7535 (
Distribution
New to Sweden. The species was previously known only from Norway and Greenland (
In Norway, the species grows on sun-exposed, more or less sloping rock surfaces, often where water is trickling or near rivers or waterfalls in the subalpine and alpine regions. The Swedish locality is a boulder in the alpine region.
Specimen examined
SWEDEN. Torne Lappmark: Låktatjåkka, 68°24.87'N, 18°19.07'E, alt. 640 m, steep face of boulder in the low alpine region. 8 July 2014, Timdal 13464-1 (
Cladonia albonigra
Cladonia albonigra Brodo & Ahti. Canad. J. Bot. 74: 1152. 1996
Holotype
CANADA. British Columbia: Queen Charlotte Islands, Graham Island, 2 mi. SE of Port Clements. 1971, Brodo 18104 & Wong (CANL).
Distribution
Confirmed for Sweden. The species is reported from the province Torne Lappmark in northernmost Sweden by
Specimen examined
SWEDEN, Torne Lappmark: Låktatjåkka, 68°24.87'N, 18°19.07'E, alt. 640 m, steep face of boulder in the low alpine region. 8 July 2014, Timdal 13464-2 (
Lecidea degeliana
Lecidea degeliana Hertel. Herzogia 2: 41. 1970.
Holotype
NORWAY, Troms: [Harstad, Hinnøy], Sandtorg Nordvik, ad saxum micacei-schistosum aeneum fuscinigrum tinctum. 14 July 1953, G. Degelius (
Distribution
New to Sweden. Lecidea degeliana was described by
This small species may easily be overlooked or mistaken for a poorly developed L. fuscoatra. It is, however, a quite distinct species once discovered. The species is initially developing as a parasite on Acarospora spp. (Fig.
Lecidea degeliana has mostly been found on calcareous rocks in exposed, subalpine habitats but also on siliceous and iron-containing rocks. There seem to be several different species of Acarospora involved as hosts but they are often sterile and mostly unidentified by us. They all belong to the small brown species of Acarospora s. str., and are in one case identified as A. versicolor through sequencing of the ITS and in another case tentatively identified morphologically as A. rugulosa.
Specimens examined
NORWAY. Hedmark: Ringsaker, the islet Holmen
Nephroma helveticum
Nephroma helveticum Ach. Lich. Univ.: 523. 1810.
Lectotype
‘In montibus Helvetiae, Schleicher’ (H-
Distribution
New to Sweden. Nephroma helveticum is a cosmopolitan species complex with a wide ecological amplitude and a large morphological and chemical variation. In Europe, however, the species is very rare and appears to have declined considerably (Klepsland 2103,
On Ahlner´s locality, Borstaberget, the bedrock consists of greenstone (porphyrite) and the mountain has long southwest facing slopes with steep cliffs. Ahlner collected, together with N. helveticum, also the rare lichen Heterodermia speciosa. On the mountain Mitti-Skansberget N. helveticum was found in 2009 in two places 200 meters apart. It was found growing on conglomerate cliffs in the southwest facing precipices. Other species that were found on the cliffs were Peltigera rufescens, Lobaria scrobiculata, L. pulmonaria, Fuscopannaria leucophaea and Biatora vernalis.
Specimens examined
NORWAY. Buskerud: Nes municipality, Gardnosberget, MGRS: 32V NN 0230, 2309, alt. 300 m, east-facing, steep slope below high mountain wall, open spruce forest over rock field, on boulder. 10 September 2013, Hofton 13176 (
Peltula placodizans
Peltula placodizans (Zahlbr.) Wetmore, Ann. Missouri Bot. Gard. 57: 179. 1970.
Basionym
Heppia placodizans Zahlbr., Bull. Torrey Bot. Club 35: 299 (1908).
Holotype
U.S.A. Arizona, Tucson, Tumamoc Hill. 1908, Blumer (W, holotype, not seen).
Distribution
New to the Nordic countries. The species is widely distributed in arid areas of both the Northern and Southern Hemispheres (
In Norway, the species was found on a vertical wall of calcareous rock in a steep, west-facing hillside. The site has apparently previously been an open or sparsely wooded pasture, but is now in the process of being transformed into spruce forest. Other remarkable lichens collected at the site include Metamelanea caesiella (Th.Fr.) Henssen, Physcia dimidiata (Arnold) Nyl., Thallinocarpon nigritellum (Lettau) P.M.Jørg., and Toninia alutacea (Anzi) Jatta.
Specimen examined
NORWAY. Oppland: Sør-Fron municipality, Harpefoss, along the trail W of farm Tåkåstad towards Mt. Lundin, 61°34.95'N, 9°52.55'E, alt. 490 m. 1 Oct. 2007, Timdal 11054 (
Phacographa protoparmeliae
Phacographa protoparmeliae Hafellner. Bibl. Lich. 100: 106. 2009.
Holotype
AUSTRIA, Kärnten: Hohe Tauern, Kreuzeck-Gruppe, Kalkschieferwände in den SE-Hängen der Sensenspitze N der Turgger Alm, c. 200 m. 17 July 1978, Hafellner 603 (
Distribution
New to the Nordic countries. Phacographa was described by
Phacographa protoparmeliae (Fig.
Specimens examined
NORWAY. Sør-Trøndelag: Røros, Storwartz, at the site of the old copper-mine, 62°37.63'N, 11°31.19'E. 15 June 2012, Westberg 12-030 (
Rhizocarpon pycnocarpoides
Rhizocarpon pycnocarpoides Eitner, Jahresbericht der Schles. Gesellschaft für vaterl. Cultur 88, 2: 46. 1911.
Holotype
CZECH REPUBLIC, Krkonoše Mts, “an den alten Bergwerken im Riesengrunde“ (not seen).
Distribution
New to the Nordic countries. The species is apparently previously known only from the type locality in the Krkonoše Mts, where it was collected by Eitner about 1910 and by Kuták in 1927. We have not seen the type material, but rather one duplicate of the material distributed by Kuták in his exsiccate (Flechtensamml. Böhmen No. 520,
The species grows on rocks with a high content of iron and the thallus is rust coloured (Fig.
Rhizocarpon pycnocarpoides has been found at five localities in Norway and one in Sweden. All localities are rich in rust stained rock and most sites are in or near old copper or zinc mines.
Specimens examined
CZECH REPUBLIC. Krkonose. 1927, V. Kuták, Kuták, Flechtensamml. Böhmen No. 521 (
Sarcogyne algoviae
Sarcogyne algoviae H.Magn. Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 9(5.1): 78. 1935.
Holotype
[GERMANY, Bayern] Obere Seealpe in der Allgäuer Alpen bei Oberstdorf, c. 5000’. 1860, H. Rehm (
Distribution
New to Sweden. This is a little known species rarely reported in the literature. Sarcogyne algoviae belongs to the morphological group in Sarcogyne with a strongly carbonized margin (
We have found Sarcogyne algoviae on several localities in Scandinavia, two in the continental parts of southern Norway and two in the Abisko area in northernmost Sweden. In addition we have found a few specimens from the Swedish mountains under the name S. clavus in the herbarium
Specimens examined
NORWAY. Oppland: Dovre, Jønndalen, Mt Nonshaugen, S precipice of the mountain, NE of farm Jønndalen, alt. 700–800 m. 12 June 2008, Westberg 08-276 (
Sarcogyne hypophaeoides
MycoBank: MB 411805
Sarcogyne hypophaeoides Vain. ex H.Magn. Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 9(5.1): 84. 1935.
Lectotype
FINLAND. Tavastia australis, Luhanka, Keihäsniemi. 1873 (
Distribution
New to Norway, Sweden and Austria. Sarcogyne hypophaeoides has until now been known from the type material in central Finland and from one collection in Germany (
Sarcogyne hypophaeoides grows exclusively on siliceous rocks. We have found it on exposed, horizontal or sloping seashore rocks on the west coasts of Sweden and Norway, on lakeshores and on semi-exposed, vertical rocks or boulders in open coniferous forests. It is often growing with scattered apothecia on smooth rock surfaces or along cracks in the rock in a similar way to S. clavus and the two species have been found growing together on at least two localities. Difficulties to collect it and a superficial similarity to S. clavus are possibly reasons why this species has been overlooked. It appears to be fairly common in humid habitats in the boreal region of Fennoscandia but its distribution is incompletely known.
Specimens examined
AUSTRIA. Steiermark: Steirisches Randgebirge, Fishcbacher Alpen, im Feistriztal, ca 2 km E von Rettenegg. 14 Nov. 1998, Kocourkova & Hafellner 46366 (
Tephromela grumosa
Tephromela grumosa (Pers.) Hafellner & Cl. Roux. Bulletin de la Société Botanique du Centre-Ouest 7: 829. 1985.
Basionym
Lichen grumosus Pers., Ann. Bot. Usteri 14: 36. 1795. Nom. nov. Lichen caerulescens Pers., Ann. Bot. Usteri 11: 15. 1794. Nom. illeg. (non Lichen caerulescens Hagen 1782).
Type
Sine loc., „ad saxa arenaria (rubicunda), a Dom. Heyder primo observatus“ (Not seen).
Distribution
New to Norway. Tephromela grumosa has been expected to occur in Norway, as it is known from a number of provinces in Sweden and Finland. It occurs in West, Central, and North Europe and in Asia (
Specimens examined
NORWAY. Oppland: Lom municipality, Runningsgrende, Klypa. 61°43.41'N, 8°15.67'E, alt. 730 m. 28 June 2013, Bendiksby et al. 12357 (
Tremella lobariacearum
Tremella lobariacearum Diederich & M. S. Christ. Bibl. Lichenol. 61: 103. 1996.
Type
PORTUGAL. Madeira: Rabaçal, on Lobaria pulmonaria. 8 Apr. 1992, P. Diederich 4935 (
Distribution
New to the Nordic countries. Tremella lobariacearum was described by
Tremella includes mainly mycoparasitic taxa that grow on a wide range of fungal hosts, including lichenized hosts. However, mycologists and lichenologists in general did not look much at the lichenicolous species until the first comprehensive study by
Specimen examined
NORWAY. Hordaland: Tysnes municipality, Støle, 59°59.14'N, 05°29.84'E, alt. 60 m. 6 Apr. 2008, Asplund & Larsson (
Tremella wirthii
Tremella wirthii Diederich. Bibl. Lichenol. 61: 164. 1996.
Holotype
GERMANY. Bayern: Neu-Ulm, Holzheim, Obstgarten WSW Steinheim, MTB 7626/2. 6 Feb 1991, V. Wirth 21713 (
Distribution
New to the Nordic countries. Tremella wirthii was described by
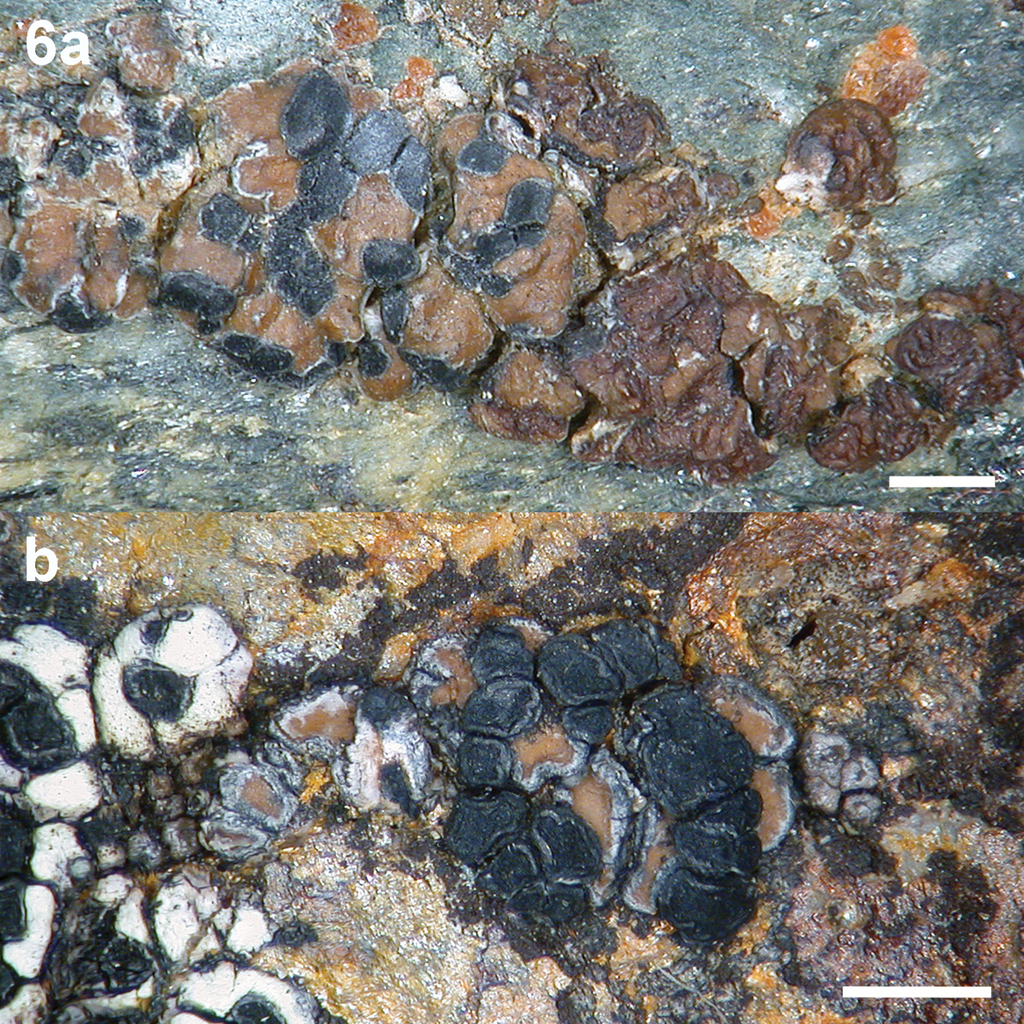
Tremella wirthii. a Basidiomata on the thallus of Protoparmelia oleagina (
Specimens examined
SWEDEN. Dalarna: Orsa municipality 61°11.25'N, 14°52.43'E, alt. 255 m. 1 Oct. 2014, Jonsson FU5955 (
Acknowledgements
M. Westberg, A. Millanes and M. Wedin are supported by grants from The Swedish Taxonomy Initiative (Svenska Artprojektet) administered by the Swedish Species Information Centre (ArtDatabanken). M. Bendiksby, R. Haugan and E. Timdal were supported by The Norwegian Taxonomy Initiative (Norske Artsprosjektet; proj no: 70184216) administered by the Norwegian Biodiversity Information Centre (ArtsDatabanken). DNA barcoding of R. pycnocarpoides and T. grumosa was funded by the Norwegian Barcode of Life project (http://www.norbol.org/). We are grateful to the curators of the herbaria mentioned for loan of material. We thank our reviewers, in particular Paul Diederich, for valuable comments that improved the manuscript.
References
- Ahti T, Stenroos S (2013) Cladonia. In: Ahti T, Stenroos S, Moberg R (Eds) Nordic Lichen Flora 5, 8–87.
- Alstrup V, Christensen SN, Skytte Christiansen M, Jacobsen P, Poulsen R, Søchting U, Svane S (1990) Notes on the lichen flora of Denmark IV. Graphis Scripta 3: 1–11.
- Alstrup V, Grube M, Motiejunaite J, Nordin A, Zhurbenko M (2008) Lichenicolous fungi from the Skibotn area, Troms, Norway. Graphis Scripta 20: 1–8.
- Alstrup V, Svane S, Søchting U (2004) Additions to the lichen flora of Denmark VI. Graphis Scripta 15: 45–50.
- Aptroot A, Diederich P, van Herk CM, Spiers L, Wirth V (1997) Protoparmelia hypotremella, a new sterile corticolous species from Europe, and its lichenicolous fungi. Lichenologist 5: 415–424. doi: 10.1017/S0024282997000509
- Aptroot A, Sparrius L, van Herk K, de Bruyn U (2001) Origin and distribution of recently described lichens from the Netherlands. Aktuelle Lichenologische Mitteilungen 5: 13–25.
- Aptroot A, van Herk CM, Sparrius LB, Spier JL (2004) Checklist van de Nederlandse korstmossen en korst-mosparasieten. Buxbaumiella 69: 17–55.
- Buschardt A (1979) Zur Flechtenflora der inneralpinen Trockentäler unter besonderer Berücksichtigung des Vinschgaus. Bibliotheca Lichenologica 10: 1–419.
- Clerc P (2004) Les champignons lichénisés de Suisse. Catalogue bibliographique complété par des données sur la distribution et l’écologie des espèces. Cryptogamica Helvetica 19: 1–320.
- Coppins BJ, Seaward MRD, Simkin J (2012) British Isles list of lichens and lichenicolous fungi. September 2012 update to list. British Lichen Society Bulletin 111: 67–69.
- Culberson CF (1972) Improved conditions and new data for the identification of lichen products by a standardized thin-layer chromatographic method. Journal of Chromatography 72: 113–125. doi: 10.1016/0021-9673(72)80013-X
- Culberson CF, Johnson A (1982) Substitution of methyl tert.-butyl ether for diethyl ether in the standardized thin-layer chromatographic method for lichen products. Journal of Chromatography 238: 483–487. doi: 10.1016/S0021-9673(00)81336-9
- Degelius G (1982) The lichen flora of the island of Vega in Nordland, northern Norway. Acta Regiae Societatis Scientiarum et Litterarum Gothobergensis, Botanica 2: 1–127.
- Diederich P (1996) The lichenicolous heterobasidiomycetes. Bibliotheca Lichenologica 61: 1–198.
- Diederich P (2003) New species and new records of American lichenicolous fungi. Herzogia 16: 41–90.
- Diederich P (2007) New or interesting lichenicolous heterobasidiomycetes. Opuscula Philolichenum 4: 11–22.
- Diederich P, Sérusiaux E (2000) The lichens and lichenicolous fungi of Belgium and Luxembourg. An annotated checklist. Musée national d’histoire naturelle, Luxembourg, 207 pp.
- Diederich P, Millanes AM, Wedin M (2014) Tremella umbilicariae (Tremellomycetes, Basidiomycota), a new lichenicolous species on Umbilicaria from Peru. Bulletin de la Société des naturalistes luxembourgeois 115: 167–172.
- Egea JM (1989) Los generos Heppia y Peltula (Liquenes) en Europa Occidental y Norte de Africa. Bibliotheca Lichenologica 31: 1–122.
- Etayo J (2002) Aportación al conocimiento de los hongos liquenícolas de Colombia. Bibliotheca Lichenologica 84: 1–154.
- Etayo J (2010) Hongos liquenícolas de Perú. Homenaje a Rolf Santesson. Bulletin de la Société Linnéenne de Provence 61: 83–128.
- Flakus A, Kukwa M (2012) New records of lichenicolous fungi from Bolivia. Opuscula Philolichenum 11: 36–48.
- Frisch A, Thor G, Ertz D, Grube M (2014) The Arthonialean challenge: Restructuring Arthoniaceae. Taxon 63: 727–744. doi: 10.12705/634.20
- Fryday A (2011) Phacographa protoparmeliae new to northern Europe from arctic Russia. Graphis Scripta 23: 21–23.
- Frödén P (2010) Liten getlav Flavoparmelia soredians ny för Norden. Lavbulletinen 2010(3): 163–165.
- Hafellner J (2009) Phacothecium resurrected and the new genus Phacographa (Arthoniales) proposed. Bibliotheca Lichenologica 100: 85–121.
- Hafellner J, Tu¨rk R (2001) Die lichenisierten Pilze Österreich – eine Checkliste der bisher nachgewiesenen Arten mit Verbreitungsangaben. Stapfia 76: 3–167.
- Haugan R, Timdal E (1994) Tephromela perlata and T. talayana, with notes on the T. aglaea-complex. Graphis Scripta 6: 17–26.
- Hertel H (1968) Beiträge zur Kenntnis der Flechtenfamilie Lecideaceae I. Herzogia 1: 25–39.
- Hertel H (1970) Beiträge zur Kenntnis der Flechtenfamilie Lecideaceae III. Herzogia 2: 37–62.
- Hertel H (1995) Schlüssel für die Arten der Flechtenfamilie Lecideaceae in Europa. Bibliotheca Lichenologica 58: 137–180.
- Himelbrant DE, Motiejunaite J, Stepanchikova IS, Tagirdzhanova GM (2014) New records of lichens and allied fungi from the Leningrad Region, Russia. V. Folia Cryptogamica Estonica 51: 49–55. doi: 10.12697/fce.2014.51.04
- Holien H, Tønsberg T (1994) The 10th meeting of the Nordic Lichen Society in Nord-Trondelag, Norway, 1993. Graphis Scripta 6: 67–75.
- Hultengren S, Malmqvist A, Arvidsson L (2011) Mörk örlav och praktsköldlav - två för Sverige nya oceaniska bladlavar. [Hypotrachyna afrorevoluta and Parmotrema chinense – two oceanic, foliose lichens new to Sweden]. Svensk Botanisk Tidskrift 105: 4–8.
- Ihlen PG, Wedin M (2008) An annotated key to the lichenicolous Ascomycota (including mitosporic morphs) of Sweden. Nova Hedwigia 86: 275–365. doi: 10.1127/0029-5035/2008/0086-0275
- James PW, White FJ (1987) Studies on the genus Nephroma I. The European and Macaronesian species. Lichenologist 19: 215–268. doi: 10.1017/S0024282987000239
- Lambley PW, Purvis OW (2009) Tephromela M. Choisy (1929). In: Smith CW, et al. (Eds) The lichens of Great Britain and Ireland. British Lichen Society, London, 875–877.
- Klepsland J (2013) Nephroma helveticum and N. tangeriense new to Norway. Graphis Scripta 25: 33–38.
- Klepsland JT, Timdal E (2010) Usnocetraria oakesiana (Parmeliaceae) new to northern Europe. Graphis Scripta 22: 14–17.
- Knudsen K, Kocourková J (2010) Lichenological notes 1: Acarosporaceae. Mycotaxon 112: 361–366. doi: 10.5248/112.361
- Knudsen K, Kocourková J, Nordin A, Sipman H (in press) Acarospora cinerascens (Acarosporaceae), a poorly known species from the southern Alps (Italia and Switzerland). Herzogia.
- Knudsen K, Kocourková J, Westberg M (2013) The identity of Sarcogyne hypophaea (Nyl.) Arnold. Opuscula Philolichenum 12: 23–26.
- Knutsson T, Johansson T (2011) Lavfloran i Ottenbylund. Länsstyrelsen, Kalmar. Meddelandeserien 2011: 13.
- Kubiak D, Zaniewski P, Wrzosek M (2010) Notes on the distribution of Sphinctrina anglica and its host in Poland. Polish Botanical Journal 55: 239–242.
- Kukwa M, Lubek A, Szymczyk R, Zalewska A (2012) Seven lichen species new to Poland. Mycotaxon 120: 105–118. doi: 10.5248/120.105
- Magnusson AH (1924) New species of the genus Acarospora. Svensk Botanisk Tidskrift 18: 329–342.
- Magnusson AH (1926) New or misunderstood European lichens. Acta Horti Gothoburgensis 2: 71–82.
- Magnusson AH (1935) Acarosporaceae, Thelocarpaceae. In: Rabenhorst GL (Ed.) Kryptogamen-Flora von Deutschland, Österreich, und der Schweiz. 2nd IX. Die Flechten. Abt. 5(1). Borntraeger, Leipzig, 1–318.
- Menlove JE (1974) Thin-layer chromatography for the identification of lichen substances. Bulletin of the British Lichen Society 34: 3–5.
- Millanes AM, Westberg M, Wedin M, Diederich P (2012) Tremella diploschistina (Tremellomycetes, Basidiomycota, Fungi), a new lichenicolous species growing on Diploschistes. Lichenologist 44: 321–332. doi: 10.1017/S0024282911000788
- Millanes AM, Diederich P, Westberg M, Knutsson T, Wedin M (2014) Tremella rhizocarpicola sp. nov. and other interesting lichenicolous Tremellales and Filobasidiales in the Nordic countries. MycoKeys 8: 31–41. doi: 10.3897/mycokeys.8.8176
- Nordin A, Moberg R, Tønsberg T, Vitikainen O, Dalsätt Å, Myrdal M, Snitting D, Ekman S [date of consultation 2015-09-08]. Santesson’s checklist of Fennoscandian lichen-forming and lichenicolous fungi. Museum of Evolution, Uppsala University. http://130.238.83.220/santesson/home.php
- Palice Z, Guttová A, Halda JP (2006) Lichens new for Slovakia collected in the National Park Muránska planina (W Carpathians). In: Lackovicová A, Guttova A, Lisicka E, Lizon P (Eds) Central European lichens – diversity and threat. Mycotaxon Ltd., Ithaca, 179–192.
- Pippola E, Kotiranta H (2008) The genus Tremella (Basidiomycota, Tremellales) in Finland. Annales Botanici Fennici 45: 401–434. doi: 10.5735/085.045.0601
- Sérusiaux E, Diederich P, Ertz D, van den Boom P (2003) New or interesting lichens and lichenicolous fungi from Belgium, Luxembourg and northern France. IX. LejeuniaN. S.173: 1–48.
- Scholz P (2000) Katalog der Flechten und flechtenbewohnenden Pilze Deutschlands. Schriftenreihe Vegetationsk.31: 1–298.
- Svensson M, Westberg M (2010) Additions to the lichen flora of Fennoscandia. Graphis Scripta 22: 33–37.
- Sundin R (2014) Svenska artprojektet - tolv år av spännande upptäckter. Fauna och Flora 109(3): 44–47.
- Søchting U, Alstrup V (2008) Danish Lichen Checklist. Ver. 2. www.bi.ku.dk/lichens/dkchecklist
- Thell A, Alstrup V, Arup U, Bendiksby M, Feuerer T, Haugan R, Kärnefelt I, Klepsland JT, Kukwa M, Launis A, Millanes AM, Motiejunaite J, Nordin A, Prieto M, Pykälä J, Seaward MRD, Timdal E, Tsurykau A, Vitikainen O, Westberg M (2014) New or interesting lichens and lichenicolous fungi from the Vadstena area, Östergötland, Sweden. Graphis Scripta 26: 15–33.
- van den Boom PPG, Etayo J, Breuss O (1995) Interesting records of lichens and allied fungi from the Western Pyrenees (France and Spain). Cryptogamie Bryologie Lichénologie 16: 263–283.
- van den Boom PPG, Etayo J (2000) Contribution to the knowledge of lichenicolous fungi and lichens from Portugal and Spain. Österreichische Zeitschrift für Pilzkunde 9: 151–162.
- Westberg M, Millanes AM, Wedin M (2008) Tremella candelariellae – en ny lavparasiterande basidiesvamp för Sverige. Lavbulletinen 2: 74–77.
- Westberg M, Thor G (2014) Lavfloran på Stora Bornö – SLF:s höstexkursion 2013. Lavbulletinen 2014: 19–33.
- Westberg M, Millanes A, Knudsen K, Wedin M (2015) Phylogeny of Acarosporaceae (Lecanoromycetes, Ascomycota, Fungi) and evolution of carbonized ascomata. Fungal Diversity 73: 145–158. doi: 10.1007/s13225-015-0325-x
- Wetmore CM (1971) The lichen family Heppiaceae in North America. Annals of the Missouri Botanical Garden 57: 158–209. doi: 10.2307/2395109
- Wirth V, Hauck M, Schultz M (2013) Die Flechten Deutschlands. Bd. 1. Ulmer, Stuttgart, 672 pp.
- Vitikainen O (2007) Nephromataceae. Nordic Lichen Flora 3: 91–95.
- Zamora JC, Pérez-Ortega S, Rico VJ (2011) Tremella macrobasidiata (Basidiomycota, Tremellales), a new lichenicolous fungus from the Iberian Peninsula. Lichenologist 43: 407–415. doi: 10.1017/S0024282911000405