(C) 2013 Christian Printzen. This is an open access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC-BY), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
For reference, use of the paginated PDF or printed version of this article is recommended.
Ecological and historical biogeography of lichens have rarely been studied in a concerted effort, but both aspects have to be taken into consideration when explaining the distributional patterns of species. This review summarizes, partly preliminary, results from a series of studies on phylogeography, ecophysiology and symbiotic interactions of the lichen Cetraria aculeata. This species is not only widespread but also occupies a very wide ecological niche. Evidence suggests that Cetraria aculeata has evolved and diversified in the Northern Hemisphere and colonised the Southern Hemisphere during the Pleistocene. Genetic isolation of populations indicates the absence of ongoing long range dispersal and genetic exchange between geographically isolated populations. We observe a hitherto unrecognized genetic diversity that may indicate ecotypic differentiation and speciation processes. Mediterranean and Polar populations differ not only genetically, but also in ecophysiological properties. Ongoing common garden experiments will have to show whether genetically fixed adaptation or acclimation is responsible for these differences. The genetic structure of the photobiont is best explained by climatic differences between localities, but co-dispersal with the mycobiont plays an important role as well. Taken together, these results indicate that a photobiont switch in the past enabled Cetraria aculeata to widen its ecological niche, with subsequent genetic isolation of populations. Photobiont switches may play a crucial role in speciation processes of lichens. A combination of ecophysiological and phylogeographic studies with experimental approaches is necessary to better understand the reaction of lichens to changing environmental conditions.
Ecological biogeography, phylogeography, population genetics, symbiont interactions, lichens, Cetraria
Biogeography aims at explaining the distributions of species, which are basically shaped by two factors: their ecological niches and their evolutionary history. Of the two fundamental subdisciplines of biogeography (
Before molecular genetic data became available, historical biogeography relied on fossil evidence or comparisons of distributional ranges of closely related species, often in conjunction with paleoenvironmental reconstructions (e.g.
Interpreting the distributional ranges of lichens has always been a challenging endeavour. Due to the sparse fossil record of lichens, historical biogeography was largely speculative before the advent of molecular markers (see e.g. the papers by
To complicate matters further, the symbiotic nature of lichens impedes the interpretation of results. Distributional ranges may be limited not by ecological restrictions of the lichen or because of dispersal limitations of the mycobiont, but because suitable photobiont(s) are absent. Adaptations to local climatic conditions or acclimation may occur in only one or both of the symbionts. In symbiotic systems, adaptation may not even be the result of mutation and natural selection. A symbiotic host may “outsource” (
To sum up, the large ecological niches observed in some lichens may be the result of (1) the ability of one or both symbionts to acclimate to widely different ecological conditions, (2) ecotypic differentiation of populations in different parts of a species range, (3) „habitat-adapted symbiosis“ by selective association of mycobionts with different photobionts or microbial symbionts, and (4) an overly broad species concept and the presence of unrecognized, possibly cryptic, species. The results summarized in this short review are based on ongoing studies on the phylogeography, population genetics, symbiont interactions and ecophysiology of the widespread bipolar lichen Cetraria aculeata (Schreb.) Fr. (Fig. 1). It will become evident that even after years of research focussing on a single species there are more questions open than answered. „Inferring the past to predict the future“ (
Cetraria aculeata, habit.
Uncertain species delimitations can undermine population-level studies of lichens. If several unrecognized species are included in a study, the assumptions of null-models (e.g. panmixia or certain modes of range expansion) may be violated. Recognizing the presence of different species and restricting the dataset to one of them may lead to a different problem: erosion of the dataset, to an extent that may prevent meaningful statistical inferences. For example,
Species delimitation within Cetraria s. str. is not unproblematic. Cetraria aculeata and Cetraria muricata (Ach.) Eckfeldt can be extremely difficult to distinguish in the field. A molecular study by
Spanish authors have used the name Cetraria steppae for morphologically distinct, vagrant forms of a Cetraria with thickened and sometimes almost foliose, unbranched thalli. Apparently, norstictic acid has not been reported from this Spanish material. In a detailed anatomical, physiological and population genetic study,
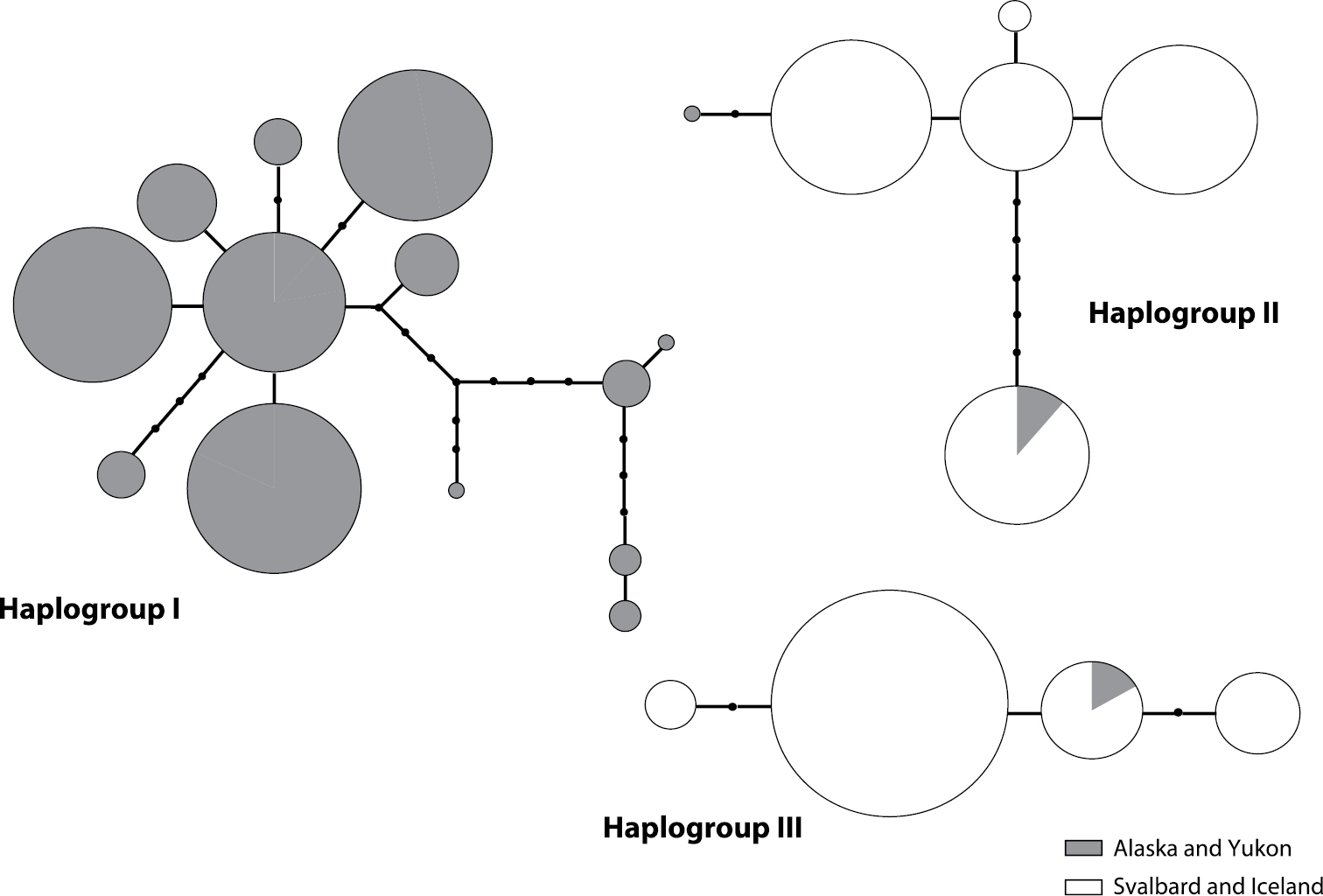
95 % parsimony probability haplotype network based on ITS sequences of the mycobiont displaying genetic differences between Beringian samples of the Cetraria aculeata group (grey) and individuals from two haplotype groups present on Iceland and Svalbard (white). Large circles represent haplotypes, each line a mutational step and black dots missing haplotypes. The size of the circles is proportional to the number of individuals sharing that haplotype. The presence of three unconnected haplogroups indicates that the genetic differences between them are too large to find a connection with a 95 % probability of being the most parsimonious one.
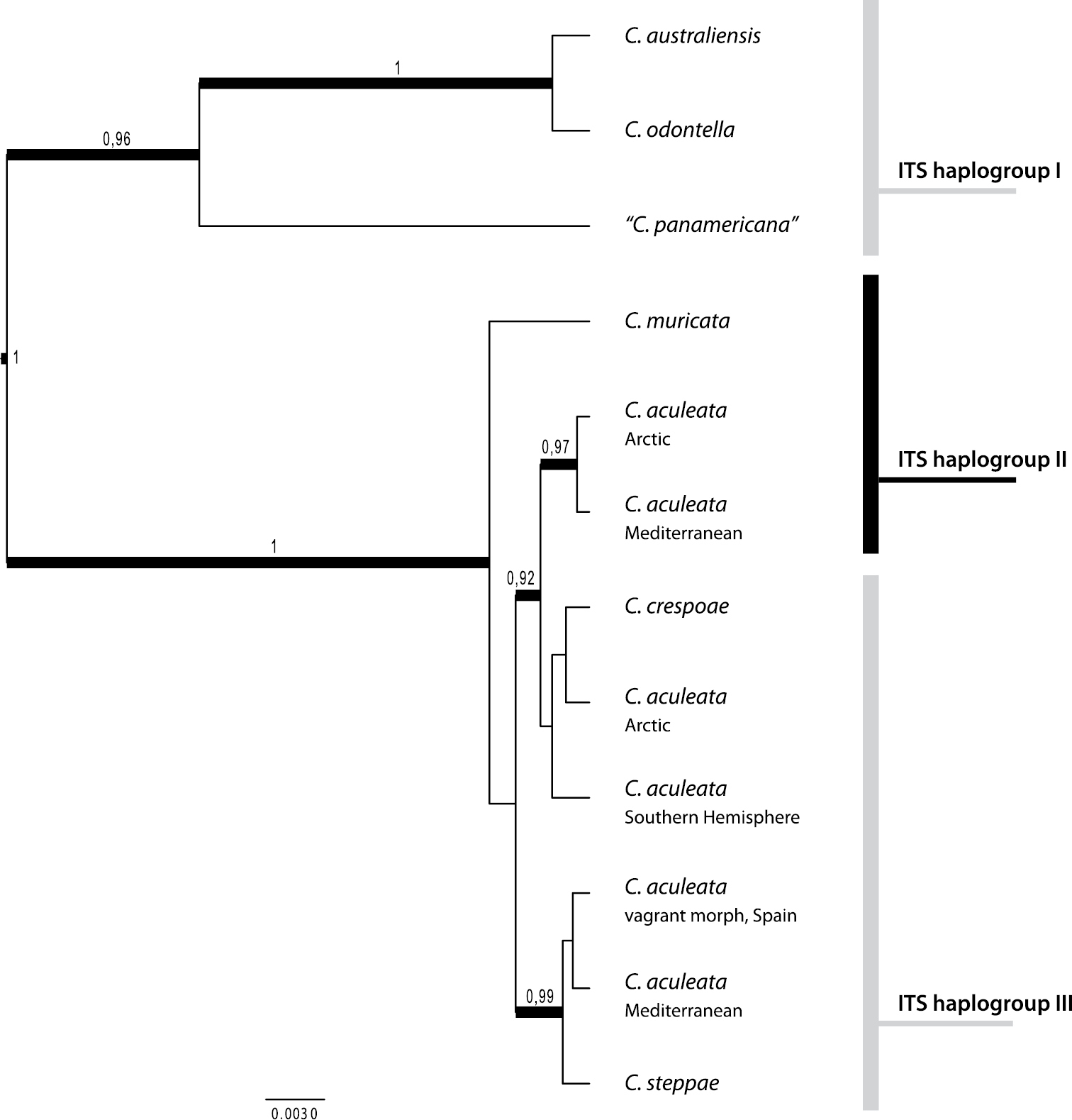
So, how many species can we distinguish within the Cetraria aculeata group and how can they be delimited? The species tree in Fig. 3 is based on a dataset comprising ITS, mtLSU and glyceraldehyde-3-phosphate dehydrogenase (GPD) DNA sequences and contains individuals of all currently accepted species of the Cetraria aculeata group. Preliminary studies (
Species tree of the Cetraria aculeata group inferred from ITS, GPD and mtLSU DNA sequences. For details of the analysis see under Material and methods.
With a high posterior probability, the Beringian and South American samples (“Cetraria panamericana”) are closely related to Cetraria odontella and Cetraria australiensis (Fig. 3). These two taxa are usually easily distinguished from other members of the Cetraria aculeata group on account of their flattened thallus lobes. However,
Questions about species delimitations and possible cryptic species put aside, the wide distribution of many lichens raises questions about how they managed to colonise their often enormous ranges. Cetraria aculeata is widespread in the Arctic and temperate regions of the northern hemisphere and common in Patagonia, Tierra del Fuego, some subantarctic islands and the maritime Antarctic. Furthermore, it has been reported from southeast Australia (
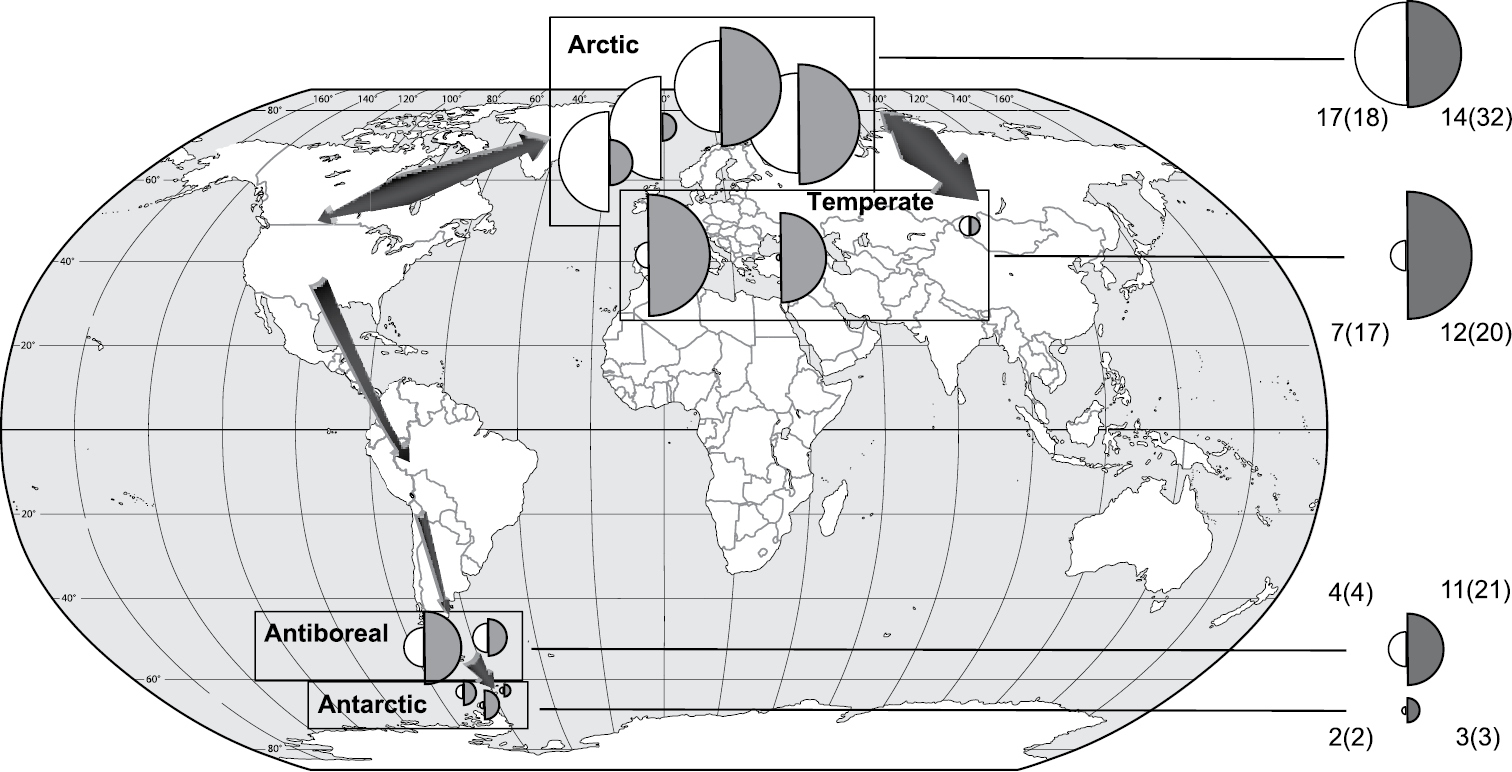
The comparison of genetic variabilities of the mycobiont of Cetraria aculeata (
Nucleotide diversity (π) of different populations of photobionts (grey) and mycobionts (white) based on ITS sequences from 222 thalli of Cetraria aculeata (data from
Because only few haplotypes are shared among both Hemispheres (see Fig. 3 in
In contrast to most other polar organisms, which are highly adapted to the extreme climatic conditions in which they live, most polar lichens are not restricted to cold habitats. Many polar species are widespread at lower latitudes, some are confined to high mountains (
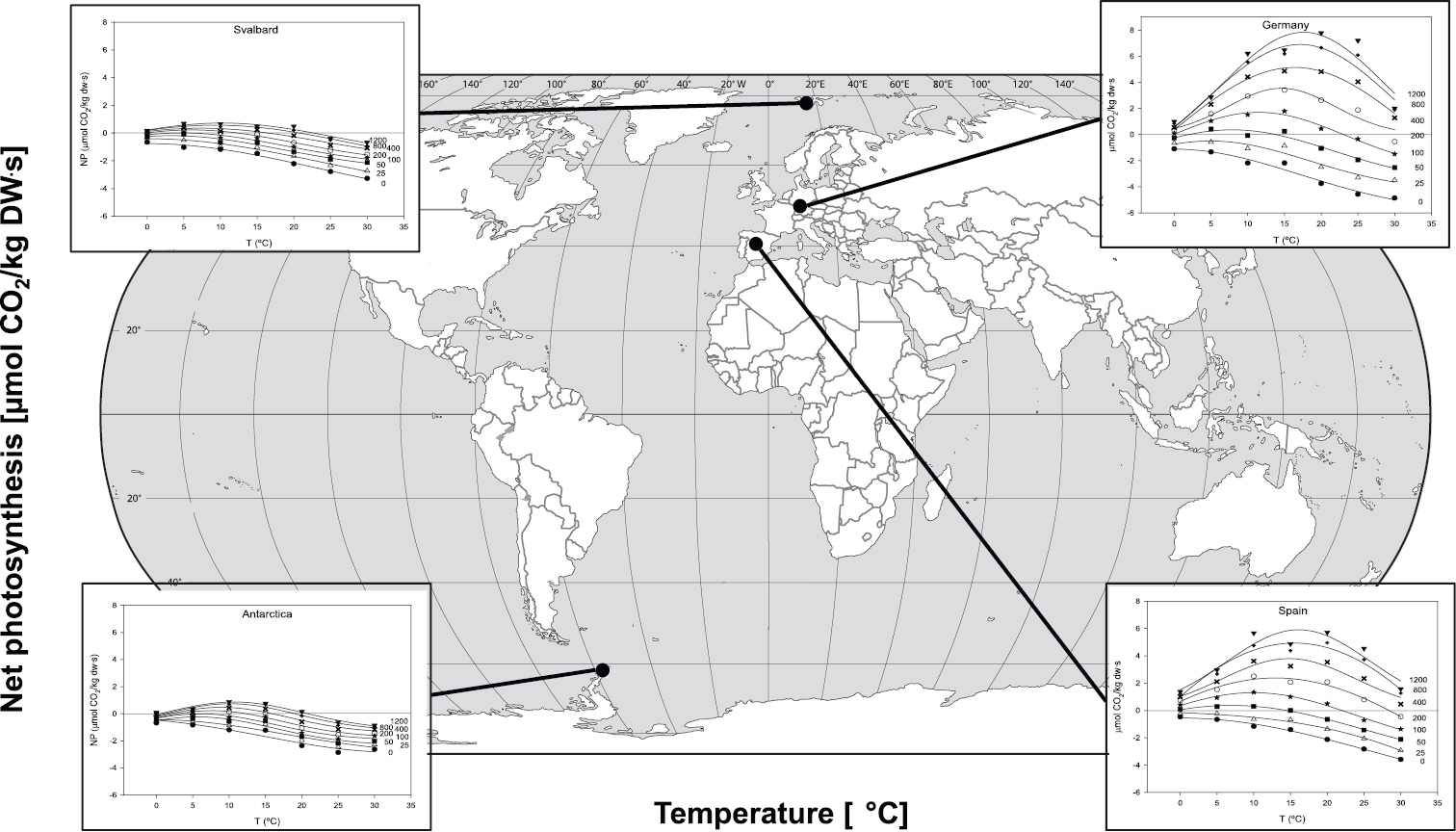
So far, the only published ecophysiological study on Cetraria aculeata is that by
Dependency of net photosynthesis (NP) on temperature at various photon flux densities (0 to 1200 µmol m-2 s-1) related to different geographical origins of Cetraria aculeata. Figures are based on measurements of 7-8 thalli per location. Further details under Material and methods.
Molecular studies have demonstrated that lichen mycobionts can associate with different photobionts (
The observation that North and South Polar populations of Cetraria aculeata are genetically more similar to each other than to the temperate populations induced
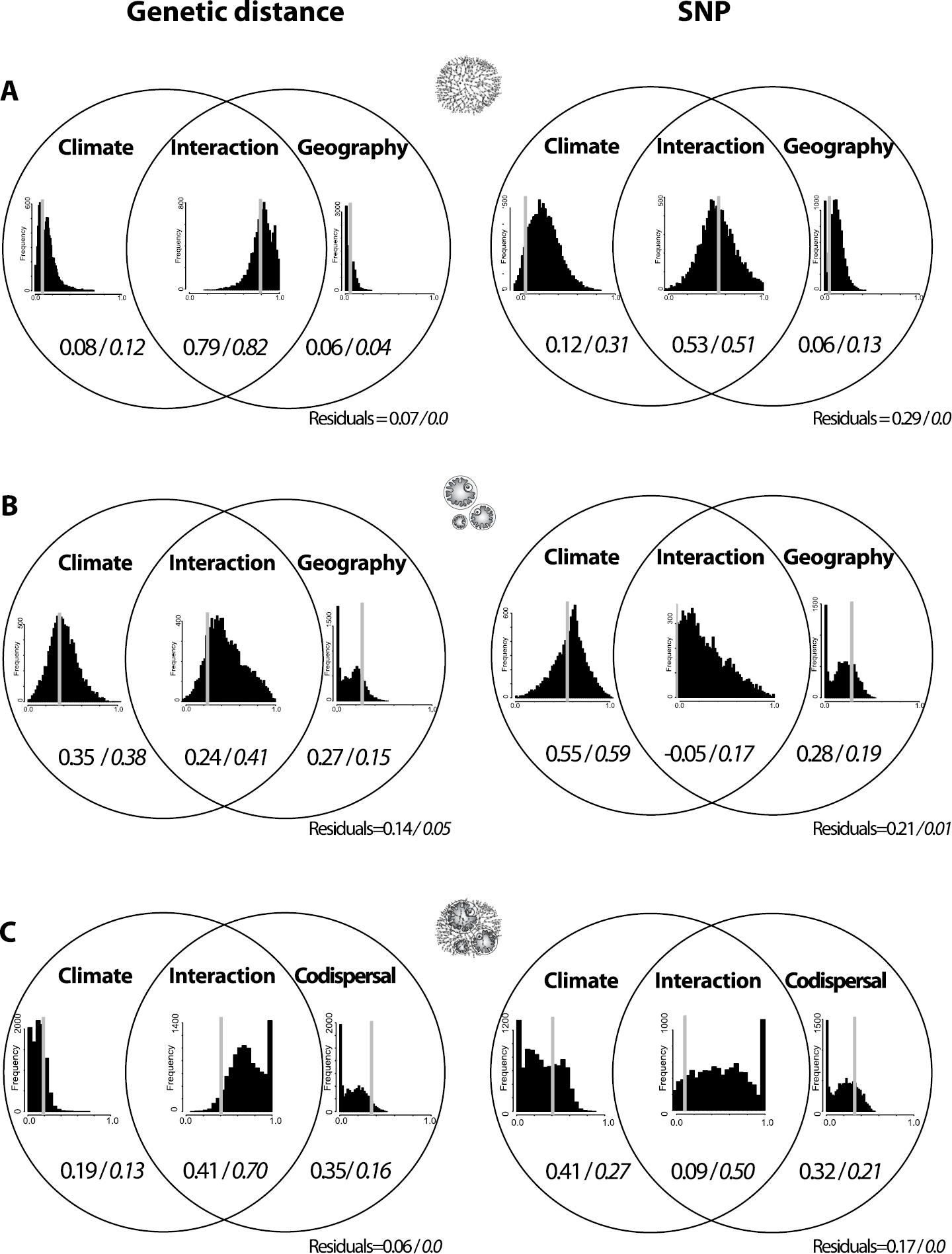
Variation partitioning diagrams using climate, geography and co-dispersal as explanatory components for the genetic structure of mycobiont and photobiont populations of Cetraria aculeata. Top, mycobiont; Center, photobiont, explanatory variables climate and spatial distances; Bottom, photobiont, explanatory variables climate and co-dispersal with the mycobiont. Left: genetic structure measured as pair-wise genetic distances between populations. Right: genetic structure measured as SNP composition. Numbers in the Venn diagrams indicate the fraction of the variation that is explained by the respective component or the intersection of components, with medians of the bootstrap analyses in italics. Results of the bootstrap analyses are also displayed in the bar charts with proportions of explained variation on the x-axes. Grey bars indicate results based on the original (non-resampled) data sets. From
It has recently become clear that the lichen symbiosis also harbours a large diversity and abundance of lichen-associated bacterial communities (reviewed by
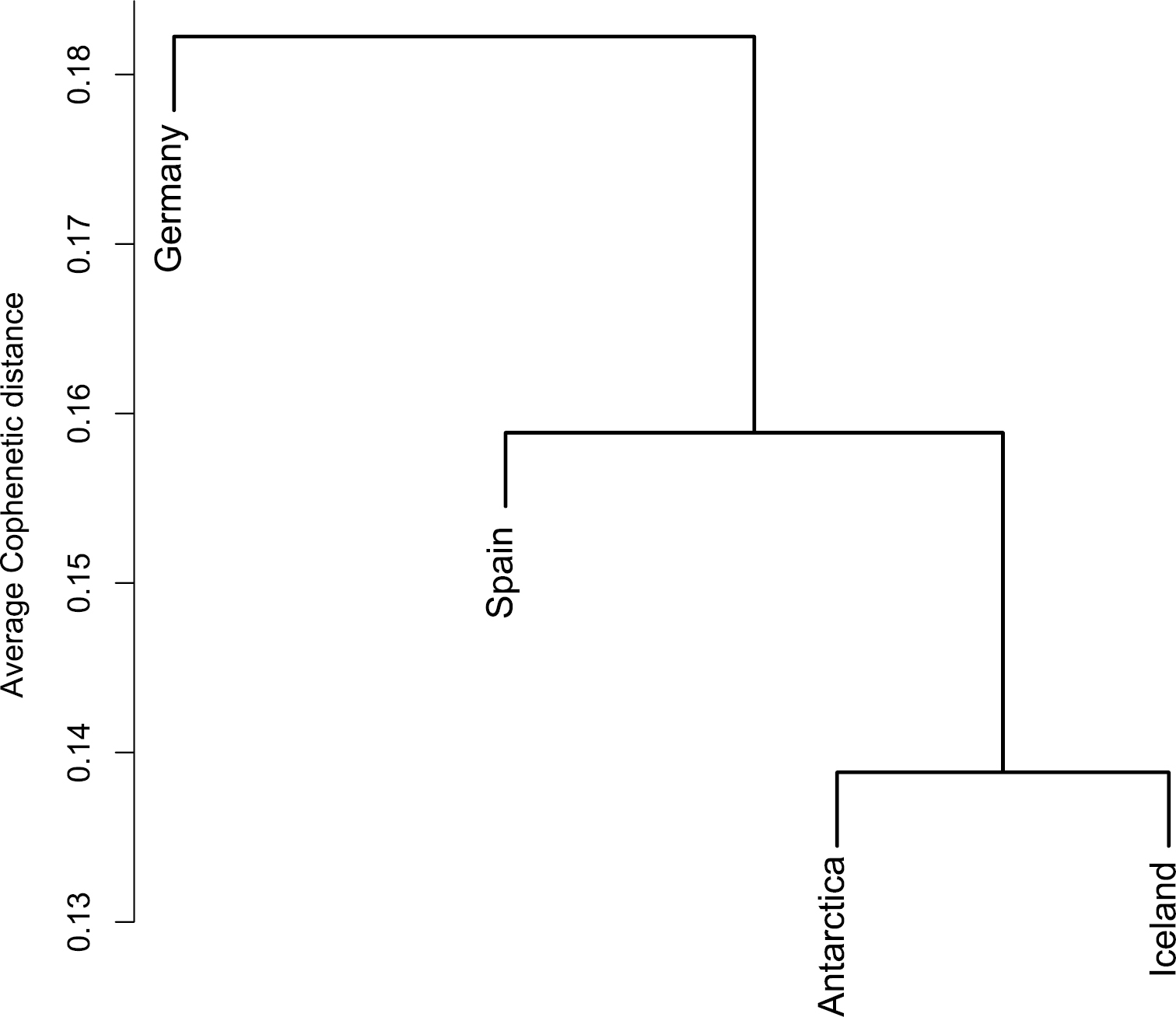
Community classification of four populations of Alphaproteobacteria associated with Cetraria aculeata. The bar on the left indicates the value of the cophenetic distances between communities. From
Sampling, methods of DNA isolation, sequencing and PCR subcloning of bacterial communities as well as statistical analyses are outlined in
Calculation of the species tree in Fig. 2 was based on 83 ITS, 71 mtLSU and 64 GPD sequences from the Cetraria aculeata group. Sequences were assigned to eleven species and genetic lineages: i) Cetraria odontella, ii) Cetraria australiensis, iii) Cetraria panamericana ined., iv) Cetraria muricata, v) Cetraria crespoae, vi) Cetraria steppae, and six geographic and genetic groups of Cetraria aculeata; vii) Mediterranean haplogroup II, viii) Mediterranean haplogroup III, ix) Southern hemisphere, x) Northern boreoarctic haplogroup II and xi) Northern boreoarctic haplogroup III. We used the species tree ancestral reconstruction method *BEAST (
CO2 exchange measurements were performed on lichen thalli that were carefully washed with mineral water and cleaned from fragments of other lichen species. Dry thalli were reactivated for 72 hours in a climate chamber (light intensity 100 µmol m-2 s –1, 12 hours light / dark cycle, 15 °C, humidification every 24 h with mineral water). CO2 measurements were performed with a compact minicuvette system (CMS 400, WALZ, Effeltrich, Germany) under controlled temperature, light and humidity conditions. For each population 7–8 replicates were investigated. Prior to all measurements, samples were soaked in mineral water for 20 minutes to ensure full hydration of thalli. Optimal water contents were determined for 4 individuals per population by studying the change of the photosynthetic performance during the desiccation process of the lichen at a constant temperature of 15 °C and photon flux densities of 0 and 400 µmol m-2 s-1. The response of net CO2 exchange was measured at optimal water content at temperatures between 0 and 30°C and photosynthetic flux densities (PPFD) of 0, 25, 50, 100, 200, 400, 800 and 1200 µmol m-2 s-1. PPFD response curves were analysed by statistical fitting of a Smith function (
Thanks are due to the co-authors of the papers and authors of theses summarized in this review: Gabriele Berg, Martin Grube and Lucia Muggia (Graz), Carmen Ascaso, Asunción de los Ríos, Míguel-Angel García, María Paz Martín, José Raggio, Leopoldo G. Sancho and Mercedes Vivas (Madrid), Patrick Jordan and Jasmin Seifried (Frankfurt). Technical support by Selina Becker, Heike Kappes (Grunelius-Möllgaard Labor, Frankfurt) and Sigrun Kraker (Graz) is gratefully acknowledged.
The studies summarized here were funded by the German Research Foundation (DFG grants Pr 567/10-1 and 13-1), the research funding programme ‘LOEWE Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz’ of Hesse’s Ministry of Higher Education, Research, and the Arts, and the Marga-und-Kurt-Möllgaard-Stiftung.